Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (6 of 92) The Schrodinger Eqn. in 1-D (2/3)
TLDRThe video script delves into the quantum mechanics concepts of energy and momentum, particularly for photons and particles with mass. It explains the derivation of equations for the energy and momentum of a photon, and then transitions to discussing the energy of a massive particle, combining kinetic and potential energy. The script highlights the leap to equating the energy of a particle with the energy of a photon, leading to the development of the Schrödinger equation. The process of deducing this fundamental equation is described as a blend of educated guessing and the need for it to align with known wave-like behaviors of particles. The video sets the stage for further exploration in the subsequent episode, where the Schrödinger equation's solutions and their implications will be discussed.
Takeaways
- 🌟 The energy of a photon is described by the equation E = H bar * Omega, where Omega is the angular frequency.
- 🚀 The momentum of a photon is given by the equation P = H bar * K, with H bar being h / 2 pi and h being Planck's constant.
- 🔍 The energy of a massive particle is the sum of its kinetic and potential energy, with kinetic energy expressed as (1/2) * m * v^2 and potential energy represented by V.
- 🌊 Small particles exhibit wave-like properties similar to photons, leading to the substitution of energy and momentum in their respective equations.
- 📝 The guessed equation for the energy of a particle with mass is E = H bar * Omega = - (H bar^2 * k^2) / (2 * m) + V, which is only valid for particles with mass.
- 🤔 The process of deriving the energy equation involved educated guessing and multiple attempts, eventually leading to a correct description of a particle's wave.
- 🧠 The Schrödinger equation was initially guessed to have a similar form to the wave equation, but with modifications to account for the particle's potential energy.
- 📐 The guessed form of the Schrödinger equation is ∂ψ/∂t = (-H bar^2 / (2 * m)) * ∂^2ψ/∂x^2 + V * ψ, where ψ is the wave function.
- 🌈 The traditional wave solutions like sin(kx - Omega t) or cos(kx - Omega t) do not work with the new equation due to the first partial derivative term.
- 🔄 The approach to finding the differential equation was to work backwards from a guessed solution format to determine the equation's structure.
- 🔗 The constants in the equations are derived from the relationship between photons and small particles, reflecting the quantum nature of reality.
Q & A
What are the three equations discussed in the video?
-The three equations discussed are: 1) The energy of a photon, written as H bar times Omega (angular frequency), 2) The momentum of a photon, written as H bar times K, and 3) An equation describing the energy of a particle with mass, which is the sum of its kinetic and potential energy.
How is the energy of a photon related to its momentum and the speed of light?
-The energy of a photon is the product of its momentum and the speed of light (E = p * c).
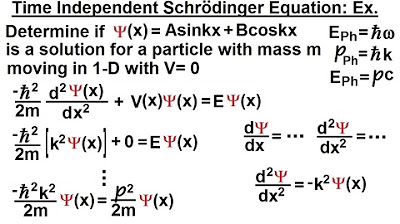
What is the kinetic energy of a particle with mass?
-The kinetic energy of a particle with mass is given by the formula (1/2) * m * v^2, where m is the mass and v is the velocity of the particle.
How does the potential energy of a particle influence its total energy?
-The total energy of a particle with mass is the sum of its kinetic energy and its potential energy, represented by the symbol V.
What is the significance of the substitution of the energy in the equation with H bar Omega?
-The substitution of the energy in the equation with H bar Omega is a leap of faith based on the idea that small particles, like photons, exhibit wave-like properties, and this allows for the description of the wave-like behavior of particles such as electrons.
Why is the energy equation for a particle with mass only valid for real particles and not for photons?
-The energy equation for a particle with mass is only valid for real particles because photons, being massless, do not have potential energy in the same sense as particles with mass.
What is the Schrödinger equation mentioned in the script?
-The Schrödinger equation is a fundamental equation in quantum mechanics that describes how the quantum state of a physical system changes with time.
How did the scientists arrive at the form of the Schrödinger equation?
-The scientists used educated guessing and worked backwards from the known solutions of the wave equation to formulate the Schrödinger equation.
What is the role of the constant H bar in the equations?
-H bar, which is Planck's constant divided by 2 pi (h / 2 pi), is a fundamental constant that appears in the equations describing the energy and momentum of photons and the energy of particles with mass.
Why did the scientists replace the momentum P with H bar k in the energy equation?
-They replaced the momentum P with H bar k to incorporate the relationship between the momentum of a particle and its wave-like properties, as derived from the de Broglie hypothesis.
What is the significance of the wave function in the context of the Schrödinger equation?
-The wave function, which is a solution to the Schrödinger equation, provides information about the probability amplitude of finding a particle in a particular state or position.
How does the video script relate to the understanding of quantum mechanics?
-The script provides a foundational understanding of the mathematical constructs and principles that underpin quantum mechanics, such as the relationship between energy, momentum, and wave-like behavior of particles.
Outlines
🌟 Quantum Mechanics Foundations: Energy and Momentum of Photons
This paragraph introduces the basic concepts of quantum mechanics, focusing on the energy and momentum of photons. It explains that the energy of a photon is given by the product of Planck's constant (H bar) and the angular frequency (Omega), and the momentum is given by H bar times the wave number (K). The discussion then transitions to finding an equation for the energy of a particle with mass, which includes both kinetic and potential energy. The kinetic energy is expressed as 1/2 m v^2, and the potential energy is represented by V. The paragraph also describes how the energy and momentum of a photon are related to the energy and momentum of a particle, leading to the development of a new equation for the energy of a particle with mass.
📚 Deriving the Schrödinger Equation: The Quantum Leap
This paragraph delves into the process of deriving the Schrödinger equation, which describes the wave function of a particle. It starts by discussing the educated guesses and the iterative process that led to the development of the equation. The paragraph explains how the energy and momentum of a particle are substituted into the equation, leading to the famous Schrödinger equation in the form of h bar^2 k^2 / 2m plus V equals the product of Planck's constant and the second derivative of the wave function with respect to position, plus the potential energy times the wave function. It also touches on the limitations of the equation, noting that it is only valid for particles with mass and not for massless particles like photons. The paragraph concludes by hinting at the next steps, which involve exploring the solutions to the Schrödinger equation and understanding why certain solutions work while others do not.
Mindmap
Keywords
💡Photon
💡Energy
💡Momentum
💡Reduced Planck Constant (ħ)
💡Angular Frequency (Ω)
💡Potential Energy (V)
💡Kinetic Energy
💡Wave Equation
💡Schrödinger Equation
💡Quantum Mechanics
💡Wave-Particle Duality
Highlights
The video discusses the derivation of equations for the energy and momentum of a photon.
The energy of a photon is given by the equation E = H bar * Omega, where Omega is the angular frequency.
The momentum of a photon is described by the equation P = H bar * K, with H bar being h / 2 pi and H being Planck's constant.
The video then explores the energy of a particle with mass, which is the sum of its kinetic and potential energy.
The kinetic energy is expressed as (1/2) * m * v^2, and the potential energy is represented by the letter V.
A leap is made by substituting the energy in the kinetic energy equation with H bar Omega, based on the wave-like behavior of small particles.
The resulting equation for the energy of a particle with mass is H bar Omega = (p^2 / 2m) + V, where p is the momentum.
This equation is only valid for particles with mass, as photons do not have potential energy.
The video introduces the Schrödinger equation as a differential equation to describe particles in one dimension.
The Schrödinger equation is hypothesized to be a linear equation, solvable as the sum of two linear equations.
The first guess for the Schrödinger equation is derived from educated guessing and the known relationship between photons and small particles.
The video explains that the traditional wave equation solutions, such as sin(kx - Omega t), no longer apply to the new equation.
A new solution format is proposed that works with the Schrödinger equation, which will be explored in the next video.
The process of deriving the Schrödinger equation is based on working backwards from a desired solution format.
The constants in the Schrödinger equation are derived from the relationship between photons and small particles.
The video sets the stage for the next installment, where the derivation and validation of the Schrödinger equation will be further explored.
Transcripts
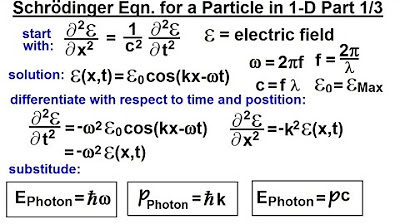
welcome to elector on line in the
previous video we were able to come up
with three equations two of them that
describe the energy of a photon the
third one that describe the momentum of
a photon energy of a photon can be
written as H bar time Omega which is the
angular frequency the energy of a photon
can be written as the product of the
momentum and the speed of light and the
momentum of a photon can be written as H
bar * K remember H bar is h / 2 pi H
being Plank's
constant next we're trying to find an
equation that describes the energy of a
particle that has mass which means the
energy will be the sum of the kinetic
energy plus the potential energy of that
particle the kinetic energy will be 1/
12 m v^ s the potential energy can be
represented by the letter
V now what we can do is we can multiply
the numerator by m and divide the
numerator by m the reason why we do that
is then in the numerator we get M * V
squared and M * V is the momentum so
this can now be written as the momentum
squared / 2 * m this would be the
kinetic energy of the particle plus the
potential energy of the
particle now since small particles act
like photons and photons have waves we
can substitute the energy in in this
equation by H bar Omega and this is kind
of a leap of Fate but hey that's what
the brogley did and he came up with the
correct equation describing the wave of
a of a small particle like an electron
so we're doing the same thing here of
course we didn't do this the first time
this was done kind of through Char and
eror so we take the energy in this
equation which is equal to P ^2 / 2m + V
and the energy is now going going to be
replaced by H bar Omega which we derived
in the previous video we're also going
to replace the momentum by H bar k which
we also derived in the previous video
which means that instead of energy we
get H bar Omega is equal to and instead
of P we have p^ S we write h bar^ 2 k^2
/ 2 M plus v so now our new equation for
the energy of a particle cannot be
written as this remember that this is
only valid for a particle because of
course we can't have a photon that has
potential energy like this so we this
can only be valid for a real particle
with mass and of course also if mass is
equal to zero we have this divided by
zero and that would be
undefined so now the next thing we're
going to do is we're going to try to
come up with a surer equation describing
this particle in one dimension we know
that the differential equation has to be
a linear solution so in other words we
should be able to write the Sur equation
as the sum of two line lar equations
like
that so what is this equation going to
look like well they took a big guess
matter of fact they probably guessed
quite a number of times try to work it
out but this was their first guess they
said well wasn't their first guess was
probably their 10th or 20 or 30 I guess
but eventually they came up with this
concept let's say that this is what the
Sher equation looks like minus H bar s /
2 m which is very similar to this except
they took away the K squ put a negative
in front of that times the second
partial derivative of the Sher equation
with respect to position plus the
potential energy of the particle times
the sh equation equals I time the first
partial derivative of the function now
you say well how did they get this well
they did some educated guessing and it
took them a while to get there so later
on we'll show you more how that can be
accomplished so one we assume that to be
the case then we realize that the old
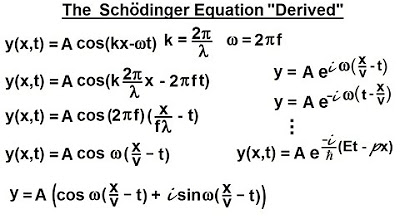
familiar equation for a wave equation
where we have the solution to be a sin
of KX - Omega t or the equation a * the
cosine of kx- Omega t no longer works
because the second part here on the
right side notice since it's the first
partial derivative you cannot get this
this as a solution this no longer works
however a solution in this form will
work with this equation and we're going
going to show you in the third video of
the of the set of three why this
equation works but we can say that if we
assume this to be the differential
equation of the shary equation here's
the Sur equation right there we can then
find the solution in this particular
format and we know that that one works
and that's part of reason why they chose
that equation they said well we're going
to assume that the equation looks like
this in its format so therefore in order
to find the differential equation that
we can find this as a solution we work
our ourselves backwards and say this
must be the format of the equation
that's really where they that's really
the way they approached it and of course
the constants came from the from the
relationship between photons and small
particles anyway so they said this is
what the solution should look like now
let's go ahead and assume that to be the
case and let's go to our next video to
see where they went at that
point
Browse More Related Video
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (5 of 92) The Schrodinger Eqn. in 1-D (1/3)

How Schrodinger Came Up With His Famous Equation (But EASIER)
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (15 of 92) Time & Position Dependencies Ex.*
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (4 of 92) The Schrodinger Eqn. "Derived"

Special Relativity | Lecture 3
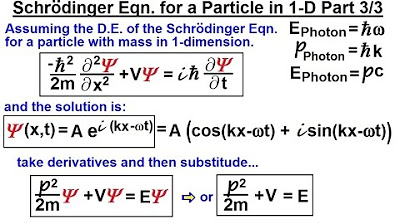
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (7 of 92) The Schrodinger Eqn. in 1-D (3/3)
5.0 / 5 (0 votes)
Thanks for rating: