Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (7 of 92) The Schrodinger Eqn. in 1-D (3/3)
TLDRIn this third part of a series, the video script explains the derivation and validation of the Schrödinger equation for a single particle in one dimension. Starting with the presumed form of the equation, the script takes the first and second derivatives with respect to time and position, leading to the canonical form of the Schrödinger equation. By substituting the expressions for momentum and energy of a photon, the script demonstrates that the derived equation simplifies to the basic energy-momentum relation, thus confirming its correctness as the proper differential equation describing a single particle's behavior in one dimension.
Takeaways
- 🌟 The series aims to derive an equation describing a single particle in one dimension.
- 📈 In the first part, three equations for a photon's energy and momentum in terms of h-bar, Omega, and K were established.
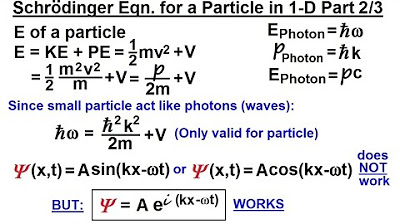
- 🎥 The second video introduced a potential form of the Schrödinger equation, e^(i(Kx - Ωt)/h-bar).
- 🔄 The third part focuses on confirming the correct form of the Schrödinger equation through mathematical derivations.
- 📚 The process involves taking the first and second derivatives with respect to time and position.
- 📶 The first derivative yields -iΩ times the original function, simplifying to -iΩψ.
- 🔢 The second derivative results in -K² times the original function, simplifying to -K²ψ.
- 🔄 By substituting these back into the presumed differential equation, the equation simplifies to h-bar squared times K squared over 2m times the function plus potential energy times the function equals h-bar Omega times i times the function.
- 🌐 This simplification aligns with the basic energy equation for a particle, confirming the Schrödinger equation's correctness.
- 📝 The final form of the Schrödinger equation for a single particle with mass m in one dimension is established.
- 🔑 The equation can be represented in differential form or as an equivalent format, both confirming the total energy of a particle as the sum of kinetic and potential energy.
Q & A
What is the main goal of the three-part series discussed in the transcript?
-The main goal is to derive an equation that describes a single particle in one dimension.
What were the three equations developed in the first part of the series?
-The three equations describe the energy and momentum of a photon in terms of h-bar Omega K P and C H bar, which involves Planck's constant divided by 2 pi.
What form did the Schrödinger equation take in the second part of the series?
-The Schrödinger equation was in the form of some constant times e to the power of another constant I times KX minus Omega T.
How does the first derivative of the Schrödinger equation with respect to time simplify?
-The first derivative simplifies to minus I Omega times the original function.
What does the second derivative of the Schrödinger equation with respect to position yield?
-The second derivative yields minus K squared times the original function.
How does the presumed Schrödinger equation relate to the total energy of a particle?
-The total energy of a particle is equal to the kinetic energy plus the potential energy, which is proven by dividing the derived equation by the Schrödinger equation and simplifying it back to the basic energy equation.
What does the momentum of the photon represent when squared and compared to the energy of the particle?
-When h-bar K is squared, it represents the momentum squared, and h-bar times Omega can be replaced by the energy of the particle, showing the relationship between momentum and energy.
What is the significance of the final form of the Schrödinger equation derived in the video?
-The final form of the Schrödinger equation represents the proper differential equation format for a single particle with mass m in one dimension, confirming its correctness.
How does the process of taking derivatives and substituting values help in validating the Schrödinger equation?
-By taking derivatives and substituting values, the process allows for the validation of the equation by showing that it simplifies back to the original energy equation, thus proving its correctness.
What is the role of Planck's constant in the equations discussed in the series?
-Planck's constant, represented by h-bar, is a fundamental constant that appears in the equations for the energy and momentum of a photon, and is also used in the Schrödinger equation to relate the particle's energy and momentum.
How does the video script demonstrate the relationship between the differential equation and its alternative format?
-The script shows that both the differential equation and its alternative format can be used to describe the single particle in one dimension, as they both lead to the same fundamental energy equation when simplified.
Outlines
📝 Introduction to the Schrödinger Equation
This paragraph introduces the third part of a series aimed at deriving an equation that describes a single particle in one dimension. It recaps the previous two parts, where three equations describing the energy and momentum of a photon were developed using Planck's constant and other constants. The focus now shifts to validating the presumed form of the Schrödinger equation by taking its first and second derivatives with respect to time and position, leading to a deeper understanding of its structure and relevance in quantum mechanics.
Mindmap
Keywords
💡Elektra Line
💡Schrödinger Equation
💡One Dimension
💡Photon
💡h-bar
💡Energy
💡Momentum
💡Derivative
💡Exponential Function
💡Potential Energy
💡Total Energy
Highlights
The series aims to develop an equation that describes a single particle in one dimension.
In the first part, three equations describing the energy and momentum of a photon were derived using h-bar Omega K P and C H bar.
The second video presented a form of the Schrödinger equation involving e to the power of (I times KX minus Omega T).
This video demonstrates that the presumed Schrödinger equation is indeed the correct form.
Derivatives of the Schrödinger equation with respect to time and position were calculated to verify its correctness.
The first derivative with respect to time yields a function multiplied by minus I Omega.
The second derivative with respect to position results in a function multiplied by minus K squared.
The results of the derivatives, when plugged back into the presumed differential equation, lead to a relation involving h-bar squared case squared over 2m times the function plus potential energy times the function.
The equation simplifies to show that the energy of the particle is equal to the momentum of the particle divided by twice the mass.
The Schrödinger equation can be rewritten using the momentum and energy of the particle.
The equation is proven correct by simplifying it back to the basic equation of total energy of a particle.
The final form of the Schrödinger equation for a single particle in one dimension with mass m is established.
The proper differential equation format of the Schrödinger equation is presented.
The process confirms the validity of the Schrödinger equation through mathematical derivation and simplification.
The video series provides a step-by-step approach to understanding the Schrödinger equation.
The highlights showcase the development and verification of the Schrödinger equation in a clear and concise manner.
The series is a valuable resource for those interested in quantum mechanics and the mathematical foundations of particle physics.
Transcripts
Browse More Related Video
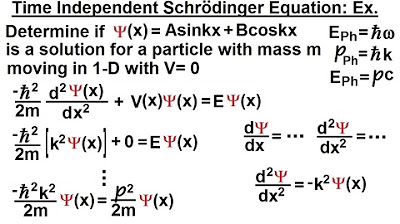
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (15 of 92) Time & Position Dependencies Ex.*
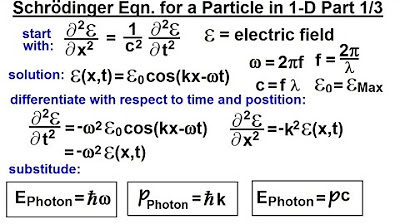
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (5 of 92) The Schrodinger Eqn. in 1-D (1/3)
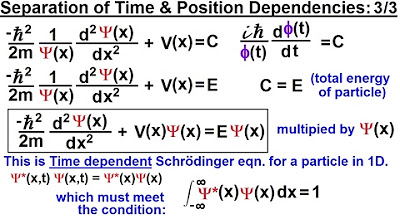
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (14 of 92) Time & Position Dependencies 3/3
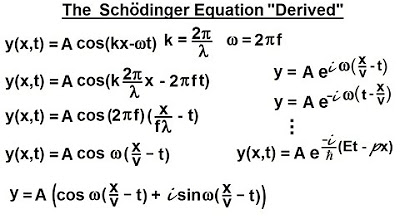
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (4 of 92) The Schrodinger Eqn. "Derived"
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (6 of 92) The Schrodinger Eqn. in 1-D (2/3)

Schrodinger Equation - A simple derivation
5.0 / 5 (0 votes)
Thanks for rating: