Quantum Mechanics - Part 2: Crash Course Physics #44
TLDRThe video explores strange ideas in quantum mechanics, like wave-particle duality and quantum superposition. It discusses how in 1923, Louis de Broglie proposed that not just light, but all matter has an associated wavelength. Experiments showed electrons can act as waves, supporting de Broglie's idea. The video also covers how probability plays a huge role in quantum mechanics, with Schrodinger's equation predicting the probability of finding a particle in space. Concepts like Heisenberg's Uncertainty Principle and Schrodinger's thought experiment with the cat demonstrate the counterintuitive weirdness of quantum physics, but also show it's the best way to describe the behavior of tiny particles.
Takeaways
- 😲 De Broglie proposed that wave-particle duality applies to all matter, not just light. This radical idea was correct.
- 🤯 Applying wave-particle duality to matter led to new ways to analyze tiny particles. It also helps explain quantum uncertainty.
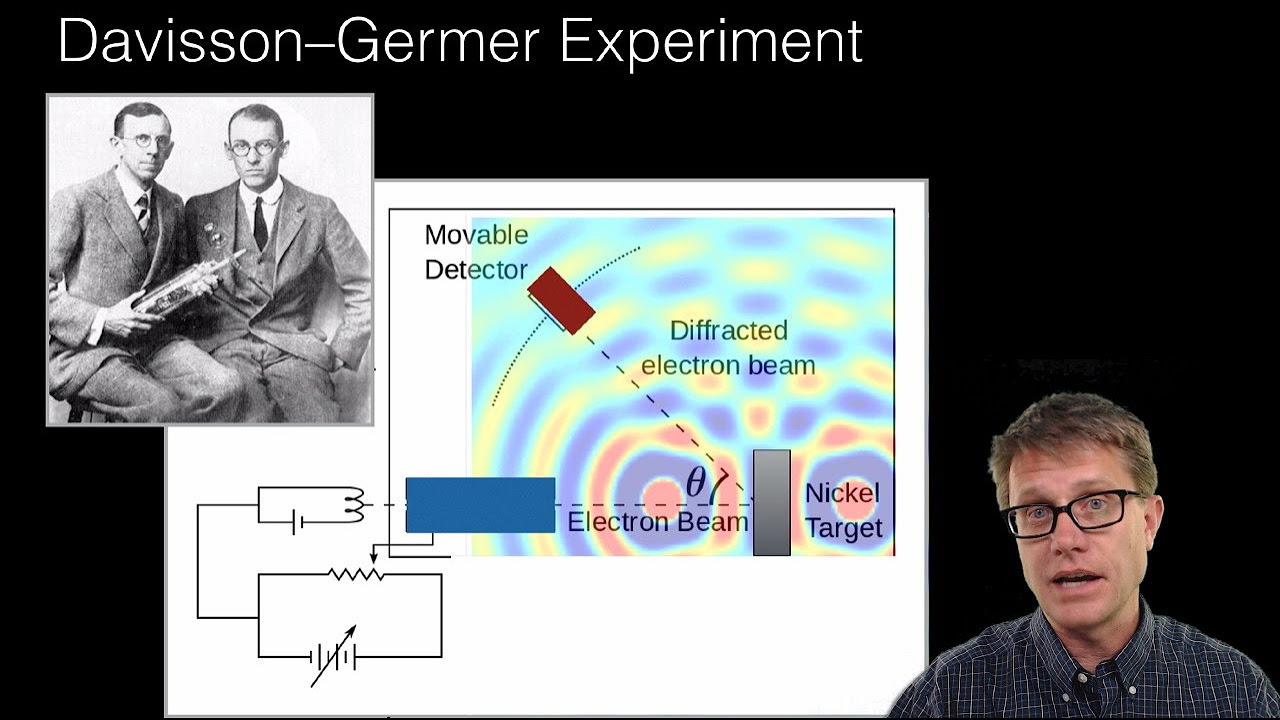
- 🌊 Electrons can create diffraction patterns like waves. This showed de Broglie was right that matter behaves like both particles and waves.
- 🔬 In quantum mechanics, probability is key to predicting the behavior of particles like electrons.
- 📏 Schrödinger's equation relates properties of quantum systems and predicts probability densities for particle locations.
- 😵💫 Quantum superposition means a particle can be in multiple states at once until it's measured.
- 🐱 Schrödinger's thought experiment with a cat aimed to show the absurdity of superposition.
- ⏱️ The Heisenberg Uncertainty Principle sets a limit on how precisely you can know a particle's position and momentum.
- 🤷♂️ Wave packets describe particles to compromise between position and momentum uncertainty.
- 🌀 Quantum mechanics gets stranger the deeper you look, but it accurately describes tiny things.
Q & A
What was Louis de Broglie's proposal about the wave-particle duality?
-De Broglie proposed that the wave-particle duality applied not just to light, but to all matter. He suggested that all matter has an associated wavelength.
How did physicists test de Broglie's idea that matter can behave like a wave?
-Physicists tested the idea by performing a double slit experiment with a beam of electrons instead of light. They found that the electrons created a diffraction pattern like light does, showing that they were behaving like waves.
Why don't we observe the wave properties of macroscopic objects?
-The wavelengths of macroscopic objects are extremely small and undetectable. The wavelength gets smaller as the momentum gets larger, and large objects like a baseball have huge momentum compared to tiny particles like electrons.
What does the wavefunction describe in quantum mechanics?
-The wavefunction describes the probability of finding a particle at any given point in space. It is used to calculate the probability density function which shows the chances of a particle being in various locations.
What does quantum superposition mean?
-Quantum superposition is the idea that a particle can be in more than one state at the same time before it is measured. For example, an electron can be thought of as being in multiple locations in a box until it is observed.
What is the Heisenberg Uncertainty Principle?
-The Heisenberg Uncertainty Principle states that there is a fundamental limit to how precisely you can know both the position and momentum of a particle at the same time. The more you know about one, the less you know about the other.
Why can't position and momentum be measured with total precision?
-When particles are described as waves, you can know the momentum precisely but the position is spread out over the wave. When described as particles, you can know the position but not the momentum. There is an inherent tradeoff.
What is a wave packet?
-A wave packet is a collection of different waves added together to describe a quantum system like an electron. It allows some idea of position while also having some uncertainty in momentum due to the wave-like properties.
Why did Schrodinger propose the cat thought experiment?
-Schrodinger proposed the example of a cat in a box being both alive and dead to demonstrate that the concept of superposition - a particle being in multiple states - is counterintuitive and even ridiculous, yet still an accurate representation.
How does quantum mechanics help describe the behavior of tiny particles?
-Quantum mechanics describes phenomena like wave-particle duality, probability waves, superposition of states, and inherent uncertainty that govern the behavior of tiny particles like electrons and atoms. Classical physics fails at this small scale.
Outlines
🤯 The weird world of quantum mechanics
This paragraph introduces the concept of wave-particle duality, explaining how light can behave as both a particle and a wave. It then discusses Louis de Broglie's proposal that this duality applies to all matter, not just light. This radical idea was proven correct - applying wave-particle duality to matter led to new ways to analyze tiny particles through quantum mechanics. However, it also leads to inherent uncertainty when measuring small particles.
👋 Understanding the quantum world
This paragraph dives deeper into the quantum concepts introduced previously. It covers topics like electron diffraction experiments, the wavefunction and Schrodinger's equation for determining probability, the intense debates around what probability means for particle location, superposition and the famous Schrodinger's cat thought experiment, and the Heisenberg Uncertainty Principle that limits how precisely we can know certain particle properties.
Mindmap
Keywords
💡wave-particle duality
💡diffraction pattern
💡momentum
💡wavelength
💡wavefunction
💡probability density
💡quantum superposition
💡Heisenberg Uncertainty Principle
💡wave packet
💡state
Highlights
Light can be both a particle and a wave, an idea proposed by quantum physics
The wave-particle duality was extended to all matter by Louis de Broglie in 1923
Experiments confirmed de Broglie's theory that electrons and all matter have wave properties
The wavelength of ordinary objects is extremely small and undetectable
Schrödinger's equation relates properties to predict the probability distribution of particles
Electron clouds show the probability distribution of electrons around an atom
Particles exist in multiple states simultaneously until measured, an effect called quantum superposition
Schrödinger proposed his famous thought experiment with a cat to show the absurdity of superposition
The Heisenberg Uncertainty Principle states position and momentum can't be precisely measured
There is a tradeoff between measuring position vs momentum due to the wave-particle duality
Physicists use wave packets to describe particles and account for uncertainty
Quantum effects like uncertainty are negligible at larger scales
De Broglie proposed that all matter has an associated wavelength
Quantum mechanics shows there are inherent limits to what we can know
Quantum physics reveals strange realities that defy everyday experience
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: