Are Photons & Electrons Particles or Waves? Make up your mind god!
TLDRThe video script explores the concept of quantum mechanics, particularly the wave-particle duality of matter. It explains how particles like photons and electrons exhibit both wave-like and particle-like properties, as demonstrated by the double-slit experiment. The script also delves into the measurement problem and the role of the observer in quantum systems, introducing the wave function and its relation to probability. Furthermore, it discusses Louis de Broglie's hypothesis that all matter has wave-like properties, with the de Broglie wavelength explaining why macroscopic objects do not exhibit quantum behavior. The video ends with a teaser suggesting a deeper exploration of the nature of particles and matter in the quantum realm.
Takeaways
- 🌀 The apparent continuity of the universe is an illusion, similar to how a TV screen's picture is made of individual pixels seen as continuous from a distance.
- 🌡️ Max Planck's discovery of the quantum nature of energy introduced the concept that energy is made of discrete chunks, expressed by the equation E=hf, where E is energy, f is frequency, and h is Planck's constant.
- 🌊 Planck's constant (h) represents the smallest amount of energy that can be associated with a given frequency, suggesting that a particle is essentially a wave packet.
- 🎥 The documentary 'Let There be Life' on MagellanTV explores the impact of quantum mechanics on the natural world and biology, offering a free view for a limited time.
- 🔄 Louis de Broglie combined Planck's and Einstein's equations to propose that mass is essentially equal to frequency, leading to his Nobel Prize-winning idea of wave-particle duality.
- 📏 The double-slit experiment demonstrates the wave-particle duality of light and other quantum objects, such as electrons and photons, which create interference patterns when not observed.
- 🚫 The act of measurement in quantum mechanics has a profound effect on the system, changing the interference pattern to particle-like behavior, highlighting the measurement problem in physics.
- 🤔 Decoherence is a concept used to understand the apparent collapse of the wave function, but it does not fully explain the change upon measurement.
- 📱 The wave function in quantum mechanics, represented by psi, is a mathematical expression that gives the probability of finding a particle in a particular location, unlike classical mechanics which predicts exact positions.
- 🌌 All matter, including macroscopic objects, exhibits wave-like behavior, but the de Broglie wavelength of larger objects is so small that their quantum behavior is not noticeable.
- 🌈 The de Broglie wavelength equation (lambda = h/(mv)) allows us to calculate the wavelength of any particle and understand why certain particles exhibit quantum behavior.
- 🌟 The concept of particles may need redefinition, as everything can be considered a wave in a quantum field, with particles being excitations or waves in this field.
Q & A
What is the illusion mentioned in the video that our universe appears to be, and what does it actually consist of?
-The illusion mentioned is that the universe appears classical and continuous, but it actually consists of discrete chunks of energy, as shown by Max Planck's work on quantum theory.
What is Planck's famous equation and what does it signify?
-Planck's famous equation is E = hf, where E represents the energy of a photon, f is the frequency of the light wave, and h is the Planck's constant. This equation signifies the relationship between energy and frequency, and introduces the concept that energy is quantized in discrete packets called photons.
How does the concept of a wave packet relate to the understanding of particles in quantum mechanics?
-A wave packet is a representation of a particle in quantum mechanics. It suggests that particles, represented by the energy they carry, can also be considered as waves. This dual nature of particles is a fundamental concept in quantum mechanics.
What is the significance of Louis de Broglie's work on combining Planck's and Einstein's equations?
-Louis de Broglie combined Planck's equation (E = hf) and Einstein's mass-energy equivalence equation (E = mc^2) to propose that mass is essentially equal to frequency, which led to the concept of wave-particle duality and earned him a Nobel prize.
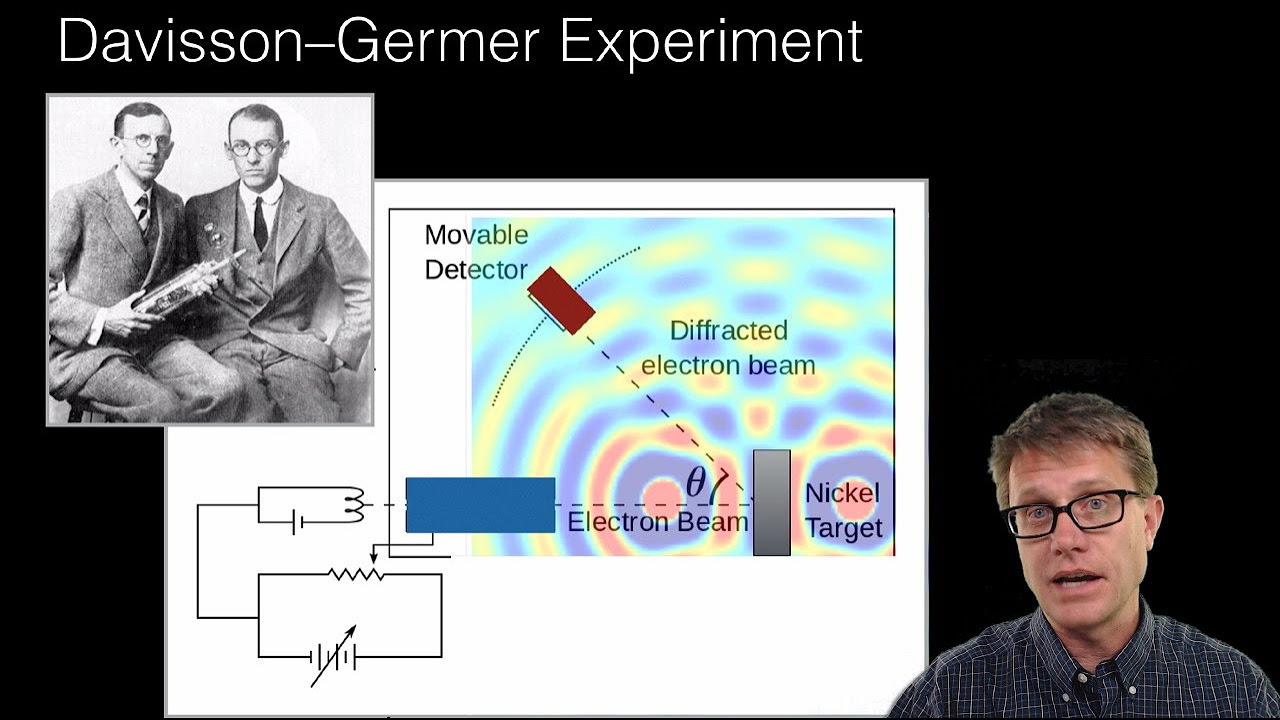
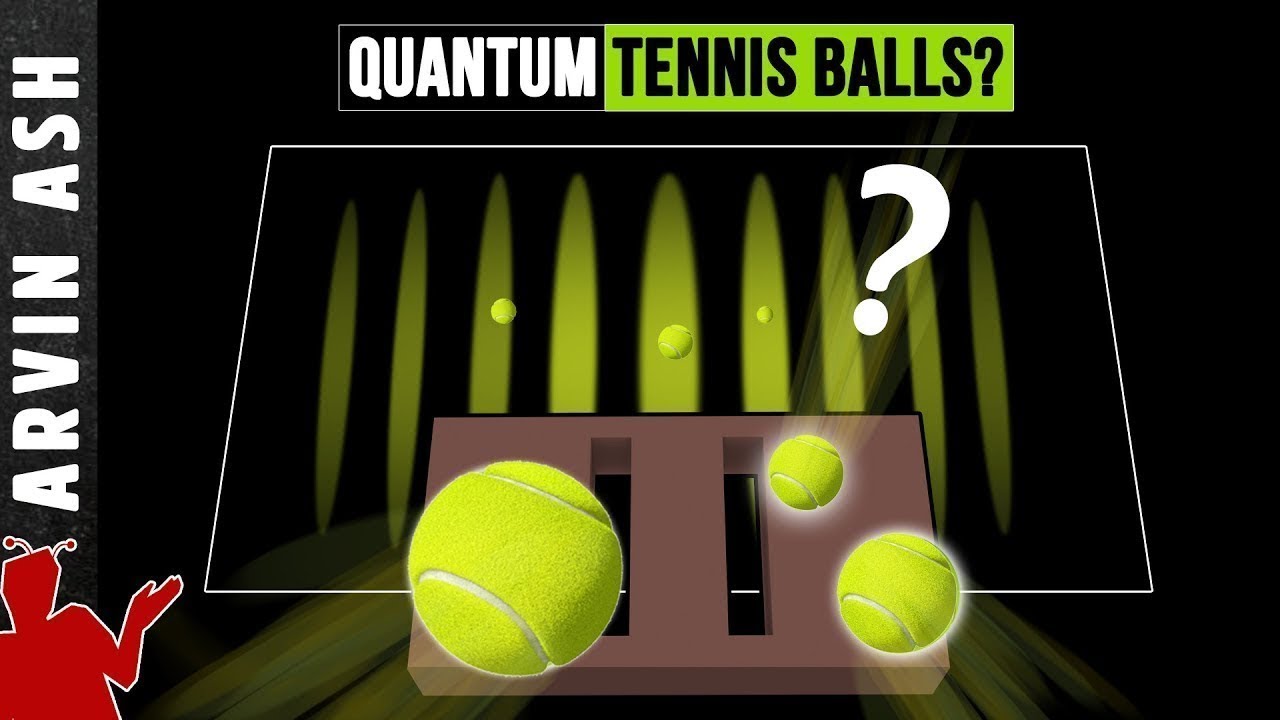
How does the double-slit experiment demonstrate the wave-particle duality of light and other quantum objects?
-The double-slit experiment shows that light and other quantum objects, like electrons and photons, exhibit both wave-like and particle-like behaviors. When these objects pass through two slits, they create an interference pattern on a screen, which is a wave phenomenon. However, when individual particles are detected, they appear as discrete particles, not waves.
What is the 'measurement problem' in quantum physics, and how does it relate to the observation of quantum objects?
-The 'measurement problem' in quantum physics refers to the phenomenon where the act of measuring a quantum object seems to change its behavior. For example, when a detector is used in the double-slit experiment to determine which slit a photon passes through, the interference pattern disappears, and the photons behave like particles rather than waves.
What is the Schrodinger equation, and how does it differ from the equations in classical mechanics?
-The Schrodinger equation is the primary equation in quantum mechanics. Unlike classical mechanics, where equations solve for a particle's position, the Schrodinger equation solves for the wave function, psi, which represents the state of a quantum system and the probability of finding the particle in a particular location.
How does the concept of wavefunction relate to the probability of finding a particle in a specific location?
-The wavefunction, represented by psi, is a mathematical expression that describes the state of a quantum system. The square of the norm of the wave function gives the probability density of finding the particle in a particular location. This means that, unlike classical mechanics, quantum mechanics does not predict exact positions but rather the likelihood of finding a particle in certain areas.
Why do macroscopic objects like basketballs and grains of sand not exhibit wave-like behavior?
-Macroscopic objects do not exhibit wave-like behavior because their de Broglie wavelengths are extremely small due to their much larger mass compared to quantum objects. The wave-like behavior is so small that it is not measurable and does not affect their macroscopic properties.
What is the de Broglie wavelength, and how can it be used to understand the wave-like behavior of particles?
-The de Broglie wavelength is a concept that describes the wavelength of a particle based on its mass and velocity. It is calculated using the equation lambda = h/(mv), where h is Planck's constant, m is the mass, and v is the velocity of the particle. This wavelength helps to understand the wave-like behavior of particles and why certain particles exhibit quantum behavior while others do not.
How does the size of a particle's wavelength relate to its quantumness or wave-like behavior?
-The size of a particle's wavelength is directly related to its quantumness or wave-like behavior. Larger wavelengths compared to the size of the particle indicate more wave-like and quantum behavior, as seen in electrons and photons. Conversely, very small wavelengths, as in macroscopic objects, result in negligible wave-like behavior and appear continuous and smooth.
What is the teaser at the end of the video suggesting about the nature of particles and matter?
-The teaser suggests a deeper understanding of quantum mechanics where it implies that there are no particles in the traditional sense, and everything is a wave in a quantum field. This means that fundamental 'particles' like electrons are actually excitations or waves in this field, challenging our classical understanding of matter.
Outlines
🌌 Quantum Illusion: Pixels, Photons, and Planck's Equation
This paragraph introduces the viewer to the concept that the seemingly continuous picture on a screen is actually an illusion composed of tiny pixels, analogous to how the universe appears classical but is fundamentally quantum. It explains the famous Planck's equation (E=hf), which relates the energy of a photon to its frequency, and introduces the idea of energy being quantized into discrete chunks. The paragraph also touches on the concept of wave-particle duality and sets the stage for a deeper exploration of quantum mechanics.
📏 Wave-Particle Duality and the Double Slit Experiment
The second paragraph delves into the wave-particle duality of quantum objects, using the double slit experiment to illustrate this phenomenon. It describes how individual photons create an interference pattern when shot through two slits, suggesting wave-like behavior. The paragraph also discusses the impact of measurement on quantum systems, leading to the disappearance of the interference pattern when photons are detected at a slit. This introduces the 'measurement problem' in physics and hints at the complex nature of quantum mechanics.
🌠 The Scale of Quantum Behavior: From Electrons to Sand Grains
The final paragraph addresses the question of why macroscopic objects like basketballs and sand grains do not exhibit quantum behavior. It explains the concept of de Broglie wavelength and how it relates to the mass and velocity of particles. The paragraph clarifies that while all matter has a wave-like nature, the wavelength of macroscopic objects is so small that their quantum behavior is not observable. It concludes by challenging the notion of particles as discrete entities and suggests that everything is a wave in a quantum field, with particles being excitations within this field.
Mindmap
Keywords
💡Quantum
💡Planck's Constant
💡Wave-Particle Duality
💡Uncertainty Principle
💡Double-Slit Experiment
💡Wave Function
💡Schrödinger Equation
💡Decoherence
💡Measurement Problem
💡de Broglie Wavelength
💡Quantum Field
Highlights
The illusion of a continuous picture on a screen is actually composed of tiny individual pixels, analogous to how our universe appears classical but is fundamentally quantum.
Max Planck's famous equation (E=hf) introduced the concept of discrete chunks of energy, challenging the classical view of continuous energy.
Planck's constant (h) represents the smallest amount of energy that any given frequency of waves can carry, indicating the particle nature of energy.
The wave-particle duality is a fundamental concept in quantum mechanics, where particles like photons exhibit both wave and particle characteristics.
The documentary 'Let There Be Life' on MagellanTV explores the impact of quantum mechanics on the natural world and biology.
Louis de Broglie's work on combining Planck's and Einstein's equations led to the idea that mass is essentially equal to frequency, earning him a Nobel prize.
The double-slit experiment demonstrates the wave-particle duality by showing that particles like photons create interference patterns as if they were waves.
The act of measurement in quantum mechanics has a profound effect on the system, as seen in the change in the interference pattern when a detector is used in the double-slit experiment.
The wave function in quantum mechanics, represented by the Greek letter psi (ψ), is a mathematical expression that describes the state of a quantum system and the probability of finding a particle in a particular location.
Quantum objects are not fixed in space but are smeared across it, unlike macroscopic objects which have definite positions.
The de Broglie wavelength equation (λ = h/(mv)) allows us to calculate the wavelength of any particle, revealing the quantum nature of matter.
Macroscopic objects like basketballs and sand grains have such small de Broglie wavelengths that their quantum behavior is not observable.
The concept of wave-particle duality extends to all matter, suggesting that everything is a wave or excitation in a quantum field.
The famous 'measurement problem' in physics highlights our limited understanding of how measurement affects quantum systems.
Decoherence is a concept used to understand the apparent collapse of the wave function, although it does not fully explain the process.
The uncertainty principle and other quantum phenomena have implications for many aspects of biology, as explored in the documentary 'Let There Be Life'.
The video teaser suggests that in future content, the nature of particles and matter will be further examined, challenging our conventional understanding.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: