What is Wave Particle Duality?
TLDRThe story of wave-particle duality unfolds through centuries of scientific inquiry, beginning with the nature of light. From ancient theories to Newton's particle view and Huygens' wave theory, the understanding of light deepens with time. Thomas Young's interference experiments and Maxwell's electromagnetic theory further challenge the particle concept. The 20th century brings a paradigm shift with de Broglie's hypothesis of matter waves, supported by electron diffraction experiments. This duality is a cornerstone of quantum mechanics, revealing that the probability of finding a particle is linked to wave amplitude, encapsulated in the wave function.
Takeaways
- 🌟 The concept of wave-particle duality is central to understanding light and the behavior of electrons, challenging the classical view of light as simply a wave or particle.
- 📜 Historical theories of light varied from viewing it as rays emitted from the eye to explanations involving indivisible atoms and the propagation of particles from a light source.
- 🔄 Newton's particle theory of light explained reflection and refraction but struggled with phenomena like diffraction and the conservation of mass.
- 🌊 Huygens' wave theory of light, proposing a medium called 'ether,' successfully explained reflection, refraction, and diffraction, leading to its dominance for over 150 years.
- 🎢 Thomas Young's double-slit experiment and the principle of superposition provided strong evidence for the wave theory of light, showing interference patterns that could not be explained by the particle theory.
- 🌐 Maxwell's electromagnetic theory predicted the existence of electromagnetic waves, including light, with a speed matching the measured speed of light, unifying electricity, magnetism, and light.
- 💡 Planck's quantum theory and Einstein's explanation of the photoelectric effect provided compelling evidence for the particle nature of light, with light described as quanta or photons.
- 📐 De Broglie extended the concept of wave-particle duality to matter, proposing that particles like electrons also exhibit wave-like properties, leading to the matter wave hypothesis.
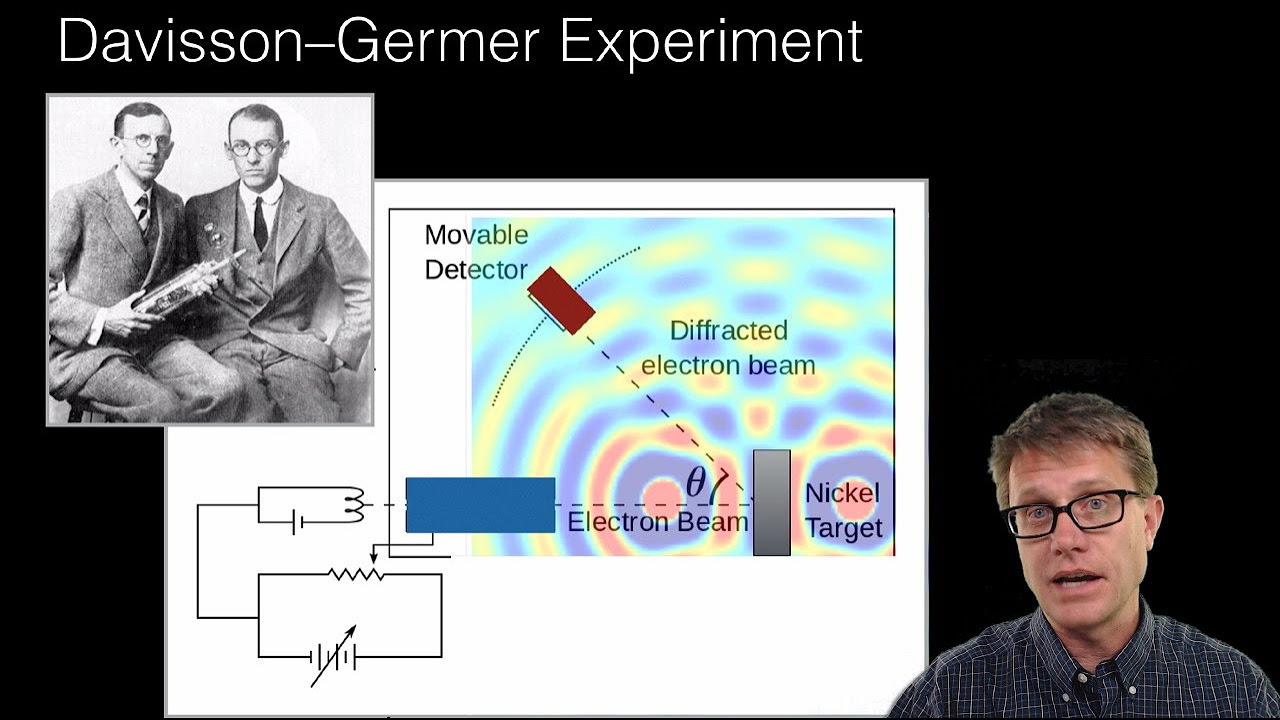
- 🔬 Experiments by Davisson and Germer, and later by George Thompson, confirmed electron diffraction, empirically validating de Broglie's hypothesis and cementing the wave-particle duality.
- 🤔 The double-slit experiment with electrons demonstrates that the probability of finding an electron at a particular location is related to the wave-like interference pattern, challenging the notion of electrons as classical particles.
- 🌀 The wave function in quantum mechanics describes the probability amplitude of finding a particle at a certain location, with the probability being equal to the square of the wave function's amplitude.
Q & A
What is the historical significance of the wave-particle duality in the context of light?
-The wave-particle duality represents a major leap in our understanding of the universe, as it challenged and expanded upon the classical views of light. It involved the synthesis of ideas across generations and the contributions of many famous scientists, leading to a revolutionary change in how we perceive the nature of light and the universe.
How did the early theories of light describe its behavior?
-Early theories of light, such as those proposed by Pythagoras and Epicurus, described light as rays emanating from the eye or objects, respectively. Later, Greek philosophers like Euclid and Ptolemy used ray diagrams to explain reflection and refraction. Lucretius and Democritus proposed that light consisted of indivisible particles traveling in straight lines, which helped explain the formation of shadows.
What were the key aspects of Ibn al-Haytham's contribution to the particle theory of light?
-Ibn al-Haytham developed the particle theory of light in his book of optics, providing a comprehensive treatment of light properties, including its physiological effects on the eye and the behavior of light when interacting with lenses and mirrors. His work laid the foundation for understanding light's behavior in various conditions.
How did Newton's particle theory of light explain reflection and refraction?
-Newton's particle theory of light explained reflection as the bouncing back of light particles at the same angle at which they hit a surface, similar to a ball's reflection. Refraction was explained by the assumption of a force of attraction between matter and light, causing a change in the horizontal component of the light particle's velocity as it enters a new medium, thus changing its direction towards the normal.
What were the main challenges faced by Newton's particle theory of light?
-Newton's particle theory faced challenges such as requiring light to travel faster inside a medium than in air, predicting a continuous loss of mass by luminous objects, and struggling to explain the diffraction of light through a narrow slit.
How did Huygens' wave theory of light differ from Newton's particle theory?
-Huygens' wave theory proposed that light was a longitudinal wave requiring a medium (ether) to travel through, and it explained reflection, refraction, and diffraction by the propagation of wave fronts and the principle of superposition, where wavelets from different points combine to form new wavefronts and interfere with each other.
What was the significance of Thomas Young's double-slit experiment in the context of the wave theory of light?
-Thomas Young's double-slit experiment demonstrated the interference properties of light, which supported the wave theory. The observed pattern of light and dark fringes could only be explained by the principle of superposition, indicating that light behaves as a wave, with the interference of overlapping waves producing the observed pattern.
How did Maxwell's equations contribute to our understanding of light as an electromagnetic disturbance?
-Maxwell's equations provided a mathematical description of the relationship between electric and magnetic fields, predicting the existence of oscillating electromagnetic waves with a speed that matched the measured speed of light. This suggested that light is an electromagnetic disturbance propagated through the field according to electromagnetic laws.
What was the ultraviolet catastrophe, and how did it lead to the development of quantum theory?
-The ultraviolet catastrophe was a theoretical problem that arose when combining electromagnetic theory with statistical laws of thermodynamics, predicting an infinite amount of energy emitted by hot objects at high frequencies. This issue was resolved by Max Planck, who introduced the concept of energy being transferred in discrete quanta, which helped explain the radiation properties of hot objects and laid the groundwork for quantum theory.
How did the photoelectric effect provide evidence for the particle nature of light?
-The photoelectric effect, where electrons are emitted from a metal surface when struck by light above a certain threshold frequency, could not be explained by the wave theory. Einstein's explanation, which assumed light was composed of particles called photons, each with energy proportional to its frequency, provided a compelling evidence for the particle nature of light and won him the Nobel Prize.
What was de Broglie's hypothesis regarding matter waves, and how did it extend our understanding of wave-particle duality?
-De Broglie hypothesized that if light waves can exhibit particle-like behavior, then matter particles, such as electrons, could also exhibit wave-like properties. He proposed that for a matter particle with momentum, there is an associated wavelength, which he derived from the relationships between energy, momentum, and the speed of light. This hypothesis extended the concept of wave-particle duality to all matter, not just light.
How did the experiments of Davisson and Germer confirm de Broglie's hypothesis?
-Davisson and Germer's experiments involved scattering electrons off a crystalline solid and observing the scattered electrons. They found that maximum diffraction occurred at specific angles and energies, which could only be explained by the wave-like behavior of electrons interfering with each other, as predicted by de Broglie's hypothesis. The experimental results matched the theoretically predicted values, confirming the existence of matter waves.
Outlines
🌟 The Mystery of Light: Wave, Particle, or Both?
This paragraph delves into the historical exploration of light's nature, from ancient theories of light rays to the revolutionary ideas of scientists like Newton and Huygens. It highlights the development of the wave-particle duality concept, where light was thought to exhibit both wave-like and particle-like properties. The narrative sets the stage for a deep dive into the evolution of our understanding of light and its fundamental role in the universe.
💡 Newton's Particle Theory and Its Limitations
This section discusses the challenges faced by Newton's particle theory of light, which posited that light consists of particles emitted by luminous objects. Despite its success in explaining phenomena like reflection and refraction, the theory encountered issues when predicting the speed of light in different media and struggled to account for the observed diffraction patterns. The paragraph sets the stage for the emergence of the wave theory as an alternative explanation for light's behavior.
🌈 Huygens' Wave Theory and Young's Experiments
This paragraph introduces Huygens' wave theory of light, which proposed that light travels as waves through a medium called 'ether.' It also describes Thomas Young's groundbreaking experiments that demonstrated the interference properties of light, supporting the wave theory. Young's work laid the foundation for understanding light as a wave phenomenon, with his famous double-slit experiment showing the characteristic interference pattern that could not be explained by the particle theory alone.
🔄 The Electromagnetic Spectrum and Maxwell's Equations
This section explores the connection between electricity and magnetism, leading to Maxwell's equations that describe the electromagnetic field. Maxwell's work predicted the existence of electromagnetic waves, including light, and suggested that light is an electromagnetic disturbance. The discovery of radio waves and X-rays further supported the wave theory, but the conflict between the particle and wave theories of light remained unresolved, hinting at the need for a new understanding of light's nature.
🌠 Planck's Quantum Hypothesis and Einstein's Photoelectric Effect
This paragraph discusses the ultraviolet catastrophe and the introduction of Planck's quantum hypothesis, which proposed that energy is transferred in discrete packets or quanta. Einstein's explanation of the photoelectric effect further supported the particle theory of light, showing that light could be thought of as consisting of photons, each with a quantized energy. These developments marked a significant shift in the understanding of light and paved the way for the acceptance of light's dual nature as both a wave and a particle.
🌌 De Broglie's Matter Waves and the Wave-Particle Duality
This section introduces De Broglie's hypothesis that matter, like light, exhibits wave-like properties, leading to the concept of matter waves. De Broglie proposed a relationship between a particle's momentum and its associated wavelength, which was later confirmed experimentally. His work unified the particle and wave theories, suggesting that all matter has wave-like properties and that the wave function describes the probability distribution of finding a particle in space.
🔬 The Double-Slit Experiment and Quantum Mechanics
This paragraph describes the famous double-slit experiment, which further illustrates the wave-particle duality of electrons. The experiment showed that electrons, when fired individually at a detector through two slits, created an interference pattern similar to that of waves. This phenomenon indicated that the probability of finding an electron at a particular location is governed by a wave function, leading to the development of quantum mechanics and the realization that the nature of reality at the quantum level is fundamentally probabilistic.
Mindmap
Keywords
💡Wave-Particle Duality
💡Light
💡Interference
💡Diffraction
💡Photoelectric Effect
💡Quantum Mechanics
💡Wave Function
💡de Broglie Wavelength
💡Double-Slit Experiment
💡Maxwell's Equations
💡Ether
Highlights
The story of wave-particle duality begins with the enigmatic nature of light, which has been central to human understanding and yet elusive to explanation.
Light's role in human history includes being worshipped by countless cultures and studied by philosophers and scientists across generations.
The question of light's nature—whether it is a wave, a particle, or something else—has required thousands of years of experimentation and theoretical inquiry.
Early theories of light from India and ancient Greece described it as rays, with philosophers like Pythagoras and Epicurus proposing different mechanisms for vision.
Euclid and Ptolemy used ray diagrams to explain light's reflection and refraction, while Lucretius and Democritus introduced the idea of light as particles traveling in straight lines.
Ibn al-Haytham's 11th-century work on optics was the first comprehensive treatment of light's properties, including reflection, refraction, and the physiology of the eye.
Isaac Newton's particle theory of light explained reflection, refraction, and dispersion, suggesting light as a stream of particles emitted by luminous objects.
Christian Huygens proposed light as a longitudinal wave, requiring a medium (ether) to travel through, and explained reflection, refraction, and diffraction.
Thomas Young's 1801 experiments on light interference provided strong evidence for the wave theory of light, challenging Newton's particle theory.
The wave theory's success was undermined by the ultraviolet catastrophe, which predicted an infinite amount of energy emission from hot objects at high frequencies.
Max Planck resolved the ultraviolet catastrophe by introducing the concept of energy quanta, suggesting that energy is transferred in discrete chunks or quanta.
Albert Einstein's explanation of the photoelectric effect using photons provided compelling evidence for the particle nature of light.
The photoelectric effect confirmed the existence of light's granular structure and other radiations, leading to the acceptance of light having both wave and particle properties.
Arthur Compton's explanation of X-ray scattering using the photon model further solidified the particle model's importance in physics.
Louis de Broglie proposed that matter particles, like electrons, also exhibit wave-like properties, leading to the concept of matter waves.
De Broglie's hypothesis was experimentally verified by Clinton Davisson and Lester Germer, who observed electron diffraction patterns similar to X-rays.
The wave function or probability amplitude is central to quantum mechanics, relating the probability of finding a particle to the square of a wave's amplitude.
The double-slit experiment with electrons demonstrated the wave-particle duality, showing probability distributions similar to wave interference patterns.
The de Broglie wavelength is a fundamental concept in quantum mechanics, linking the particle and wave properties of matter through Planck's constant and the particle's momentum.
Erwin Schrödinger's wave mechanics and the Schrödinger equation marked a new era in physics, providing a mathematical framework for understanding quantum behavior.
The historical development of wave-particle duality has forever changed our view of the universe, integrating the seemingly contradictory properties of light and matter into a coherent quantum framework.
Transcripts
Browse More Related Video

AT&T Archives: Matter Waves, Holden and Germer on Wave Nature and the Davisson-Germer Experiment

Wave-Particle Duality of Light

I did the double slit experiment at home
Matter as a Wave

Quantum Mechanics - Part 2: Crash Course Physics #44
Can You Capture a Light Wave? Mind-Blowing Wave-Particle Duality Experiment!
5.0 / 5 (0 votes)
Thanks for rating: