AP Chem Unit 5 Review - Kinetics in 10 Minutes!
TLDRIn this engaging review of AP Chemistry Unit 5, Jeremy Krug delves into the intricacies of Chemical Kinetics, explaining how the rate of a chemical reaction is influenced by factors such as reactant concentration, temperature, particle size, and catalyst presence. The video outlines how to determine relative rates from balanced equations, introduces rate laws and their formulation through experimental data, and demonstrates how to graphically ascertain reaction orders. It also covers integrated rate laws, half-life calculations for first-order reactions, and the concept of multi-step reactions with their respective rate laws. The role of activation energy, the Boltzmann distribution, and the Arrhenius equation in understanding reaction rates at varying temperatures is explored. The importance of reaction mechanisms, intermediates, and catalysts in influencing reaction rates is also highlighted. Krug's presentation is both informative and accessible, providing a solid foundation for students preparing for AP Chemistry examinations.
Takeaways
- 🧪 Chemical kinetics involves the study of reaction rates and the factors that affect them, including reactant concentration, temperature, particle size, and catalyst presence.
- ⚖️ Reaction rates can be compared using the stoichiometric coefficients from the balanced chemical equation, helping predict the consumption and production rates of different species.
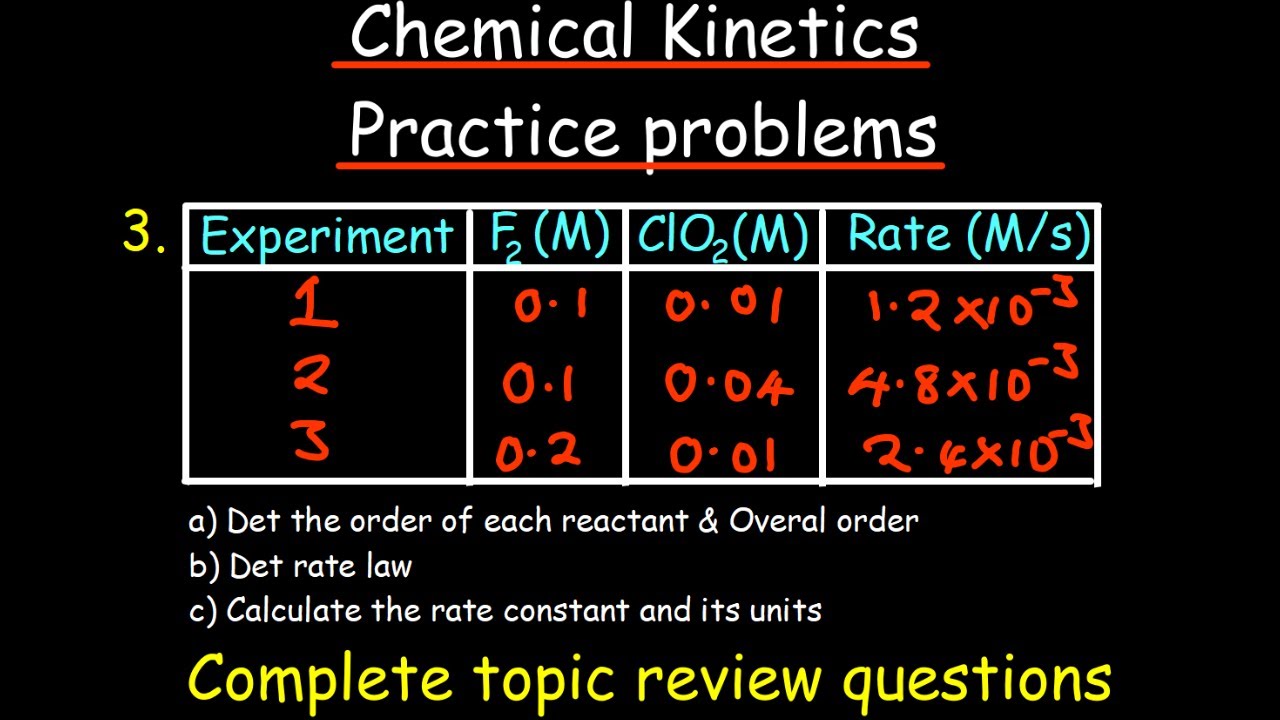
- 🔢 Rate laws relate the concentrations of reactants to the reaction's rate, typically requiring experimental data to determine the powers to which reactant concentrations are raised.
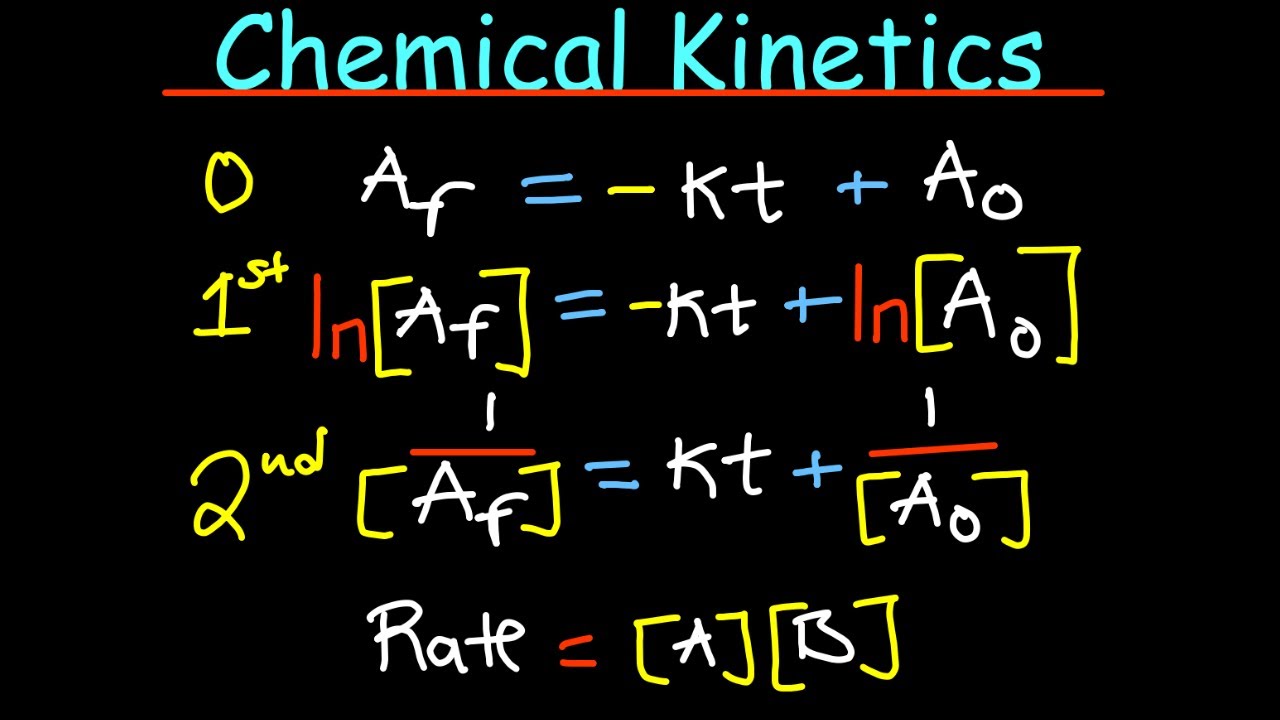
- 📊 Graphical methods can determine the order of reaction with respect to a reactant by analyzing concentration over time, using plots of concentration, its natural log, or its reciprocal against time.
- 🕒 Integrated rate laws connect the rate constant to reactant concentrations over time, allowing calculation of unknowns if three of the parameters (initial concentration, time, final concentration, rate constant) are known.
- 🔄 Multistep reactions feature individual rate laws for each step; the overall reaction rate is governed by the slowest step, which is crucial for determining the reaction mechanism.
- 🌡️ The Arrhenius equation describes how reaction rates increase with temperature by plotting the natural log of the rate constant against the reciprocal of temperature to find the activation energy.
- ⏳ Half-life calculations for first-order reactions are straightforward, involving the rate constant and the logarithmic constant (0.693).
- 🎢 Energy profiles of reactions reveal the activation energies and the changes in enthalpy, indicating whether a reaction is exothermic or endothermic.
- 💨 Catalysts accelerate reactions by lowering the activation energy and often increasing the effective collision rate between reactants.
Q & A
What is the main topic of AP Chemistry Unit 5?
-The main topic of AP Chemistry Unit 5 is Chemical Kinetics, which is about how fast or slow a chemical reaction proceeds.
What are the four main factors that affect the rate of a chemical reaction?
-The four main factors that affect the rate of a chemical reaction are the concentration of the reactants, the temperature, the particle size of the reactants, and the presence of a catalyst.
How can you determine the relative rates of reactants and products in a chemical reaction?
-You can determine the relative rates of reactants and products by looking at the coefficients of the balanced chemical equation. The ratio of the coefficients represents the ratio of the rates.
What is the typical unit used to express reaction rate?
-The typical unit used to express reaction rate is moles per liter per second (M/s), which represents the change in concentration per unit time.
What is a rate law and what does it relate?
-A rate law is an equation that relates the concentrations of the reactants to the initial rate of a chemical reaction. It is typically written in the format Rate = k[A]^x[B]^y, where k is the rate constant, [A] and [B] are the concentrations of the reactants, and x and y are the reaction orders with respect to those reactants.
How do you determine the reaction orders for each reactant in a reaction?
-To determine the reaction orders for each reactant, you need experimental data. By comparing the changes in rate with changes in concentration for different experiments, you can deduce the reaction order for each reactant.
What is the overall order of a reaction and how is it calculated?
-The overall order of a reaction is the sum of the individual orders of the reactants. It is calculated by adding up the reaction orders for each reactant involved in the reaction.
How can you calculate the rate constant for a reaction using experimental data?
-You can calculate the rate constant by taking the data from any one of the experiments, plugging it into the rate law, and solving for the rate constant k, ensuring that you use the correct units.
What are integrated rate laws and how are they used?
-Integrated rate laws are equations that relate the rate constant, initial concentration of a reactant, time elapsed, and the concentration left after that time for zero, first, and second order processes. If you know any three of these values, you can solve for the fourth.
What is the half-life equation for first-order processes?
-For first-order processes, the half-life equation is given by half-life = 0.693 divided by the rate constant.
How does a catalyst affect a chemical reaction?
-A catalyst speeds up a chemical reaction without being consumed in the process. It does this by lowering the activation energy required for the reaction and often by increasing the number of effective collisions between reactant molecules.
What is a reaction intermediate and how does it differ from a reactant in a multistep reaction?
-A reaction intermediate is a temporary molecule that is formed in one step of a reaction and used up in a subsequent step. It differs from a reactant because it is not present in the overall balanced equation of the reaction, whereas reactants are.
Outlines
🔍 Understanding Chemical Kinetics
Jeremy Krug introduces the topic of Chemical Kinetics, explaining how various factors such as reactant concentration, temperature, particle size, and the presence of a catalyst influence reaction rates. He discusses how to determine the relative rates of reactants and products from a balanced chemical equation and introduces the concept of reaction rate units. Krug also explains rate laws, how to determine the powers or orders of reactions, and the use of experimental data for this purpose. He demonstrates how to calculate the rate constant and graphically determine the order of a reactant. The integrated rate laws are also covered, along with how to derive half-life equations and the concept of multi-step reactions with their respective rate laws. The paragraph concludes with an example of a two-step chemical reaction.
📈 Reaction Mechanisms and Energy Profiles
This paragraph delves into the prerequisites for a successful molecular reaction: sufficient activation energy and correct molecular orientation. The Boltzmann distribution and its implications for reaction rates at higher temperatures are explained. A graphical representation of reaction energy from reactants to products is discussed, highlighting the activation energy and enthalpy change. The Arrhenius equation is introduced to connect rate constants with activation energy across different temperatures. The concept of reaction intermediates and catalysts is explored, with a focus on their roles in multistep reactions. The rate-determining step and its impact on the rate law of the overall reaction are explained. The paragraph concludes with a discussion on energy profiles of multistep reactions, the role of catalysts in lowering activation energy, and different types of catalysts, including surface catalysts.
Mindmap
Keywords
💡Chemical Kinetics
💡Reaction Rate
💡Rate Law
💡Reaction Order
💡Integrated Rate Laws
💡Half-Life
💡Activation Energy
💡Arrhenius Equation
💡Reaction Mechanism
💡Catalyst
💡Energy Profile
Highlights
Chemical kinetics is the study of how fast or slow a chemical reaction proceeds.
Four main factors affect reaction rate: reactant concentration, temperature, particle size, and catalyst presence.
Reaction rates can be determined from the coefficients of a balanced chemical equation.
Reaction rate is typically measured in units of concentration per unit time, like moles per liter per second.
A rate law relates reactant concentrations to the initial rate of reaction. It has the form Rate = k[A]^x[B]^y.
Experimental data is needed to determine the reaction orders (powers x and y) for each reactant.
The overall reaction order is the sum of the individual orders of the reactants.
The rate constant (k) can be calculated by plugging data from an experiment into the rate law and solving for k.
Reaction order can also be determined graphically by plotting concentration vs time, ln(concentration) vs time, and 1/concentration vs time.
Integrated rate laws relate the rate constant, initial concentration, time elapsed, and final concentration for 0th, 1st, and 2nd order reactions.
For first-order reactions, the half-life equation is t1/2 = 0.693 / k.
Many reactions occur in multiple steps, each with its own rate law. The slowest step determines the overall rate.
A reaction mechanism involves a series of elementary steps with a reaction intermediate formed and consumed.
A catalyst speeds up a reaction by lowering the activation energy and increasing the number of successful collisions.
Catalysts can be involved in the reaction mechanism, forming bonds with reaction intermediates, or act as surface catalysts.
The energy profile of a multistep reaction has multiple transition states and activation energies, with the highest peak being the slowest step.
The Arrhenius equation relates reaction rate constants at different temperatures to the activation energy.
In a reaction mechanism, the rate law is determined by the slowest step, even if it is not the first step in the mechanism.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: