Kinetics: Initial Rates and Integrated Rate Laws
TLDRProfessor Dave's video script delves into the fundamentals of chemical kinetics, explaining how reaction rates are measured and influenced by reactant concentrations. It covers concepts like instantaneous rate, reaction orders, and rate laws, illustrating how to determine the order experimentally using initial rates. The script also introduces integrated rate laws and their graphical analysis to deduce reaction orders, providing a comprehensive guide to understanding the speed and dynamics of chemical reactions.
Takeaways
- 🔍 Kinetics is the study of reaction rates, focusing on how quickly chemical reactions occur.
- ⏱ Reaction rates are measured by the increase in product concentration or the decrease in reactant concentration over time.
- 📊 Rates of change obey stoichiometry, meaning the rate of a reaction can be related to the rates of other substances involved in the reaction.
- 📈 Methods to measure reaction rates include monitoring gas pressure, light absorbance, or plotting concentration versus time.
- 📚 The instantaneous rate is calculated by the slope of the tangent line at a specific point on a concentration-time graph.
- 🔄 The rate of a reaction depends on the concentration of one or more reactants, and this relationship is described by the reaction order.
- 📝 The overall reaction order is the sum of the individual orders of the reactants involved in the reaction.
- 🔬 Rate laws are used to describe the kinetics of a reaction, with the rate constant (k) being determined experimentally.
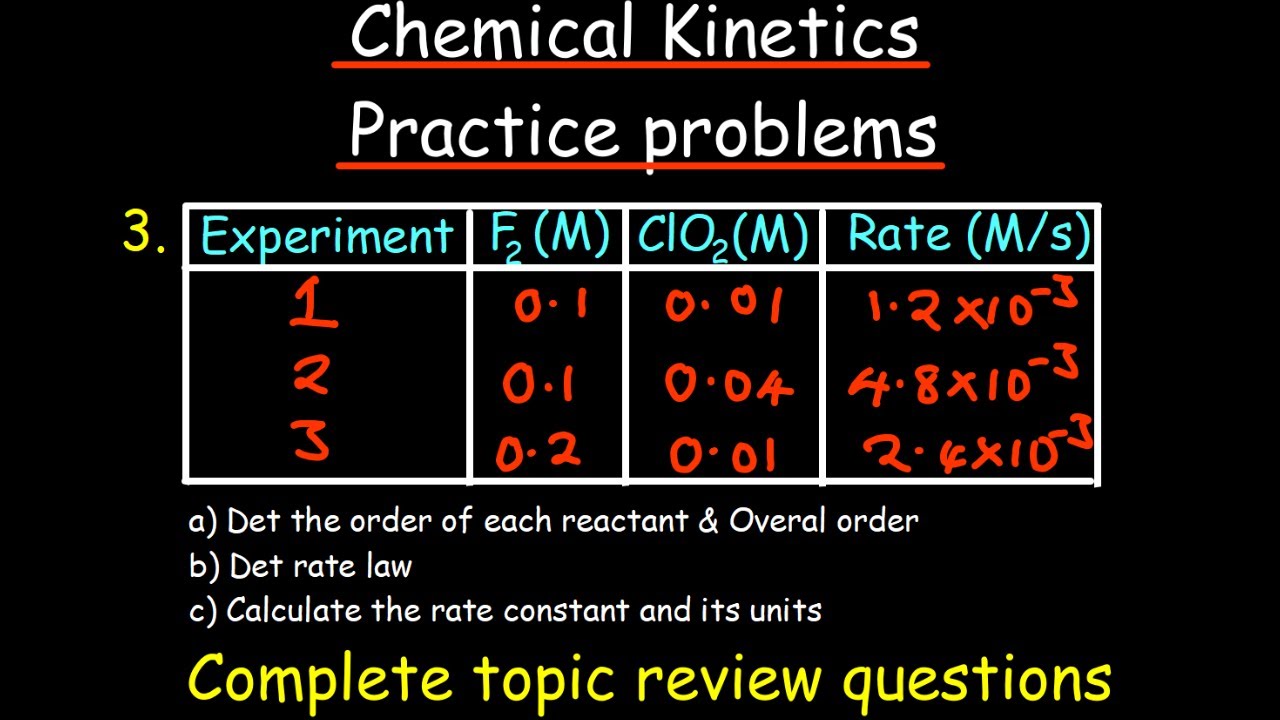
- 🧪 Initial rates data from multiple trials can help determine the reaction order with respect to each reactant by varying their initial concentrations.
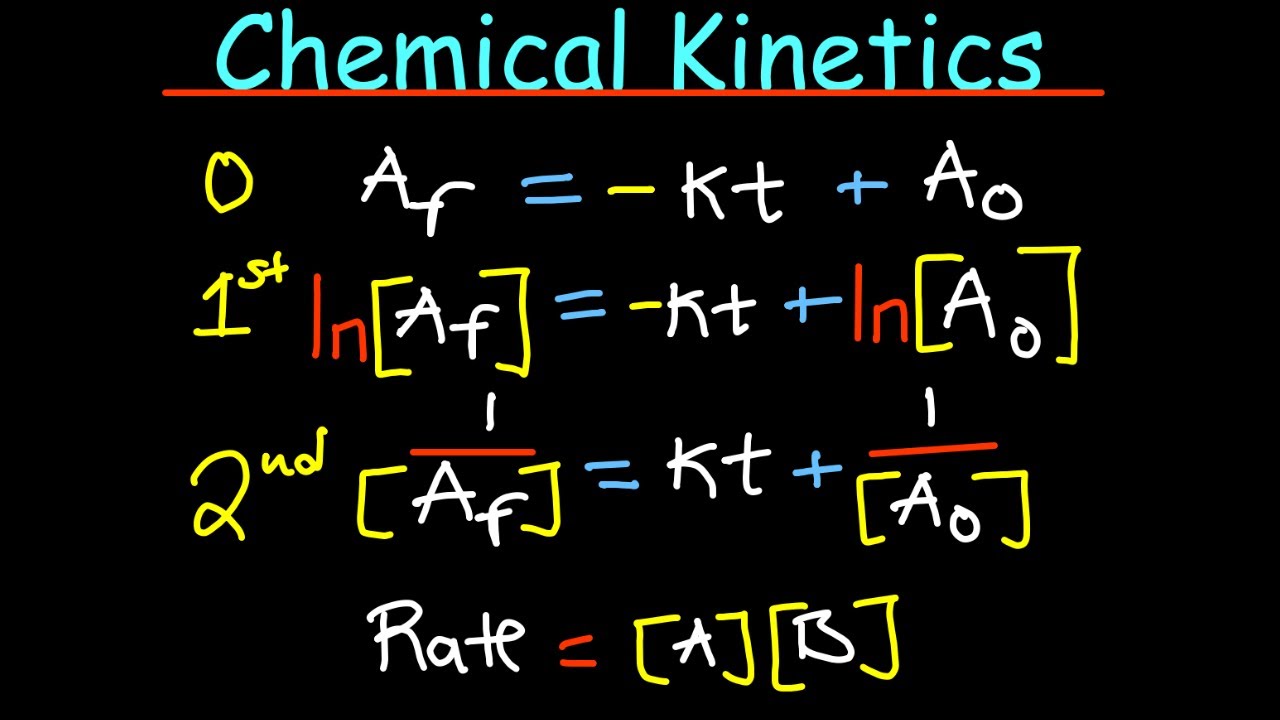
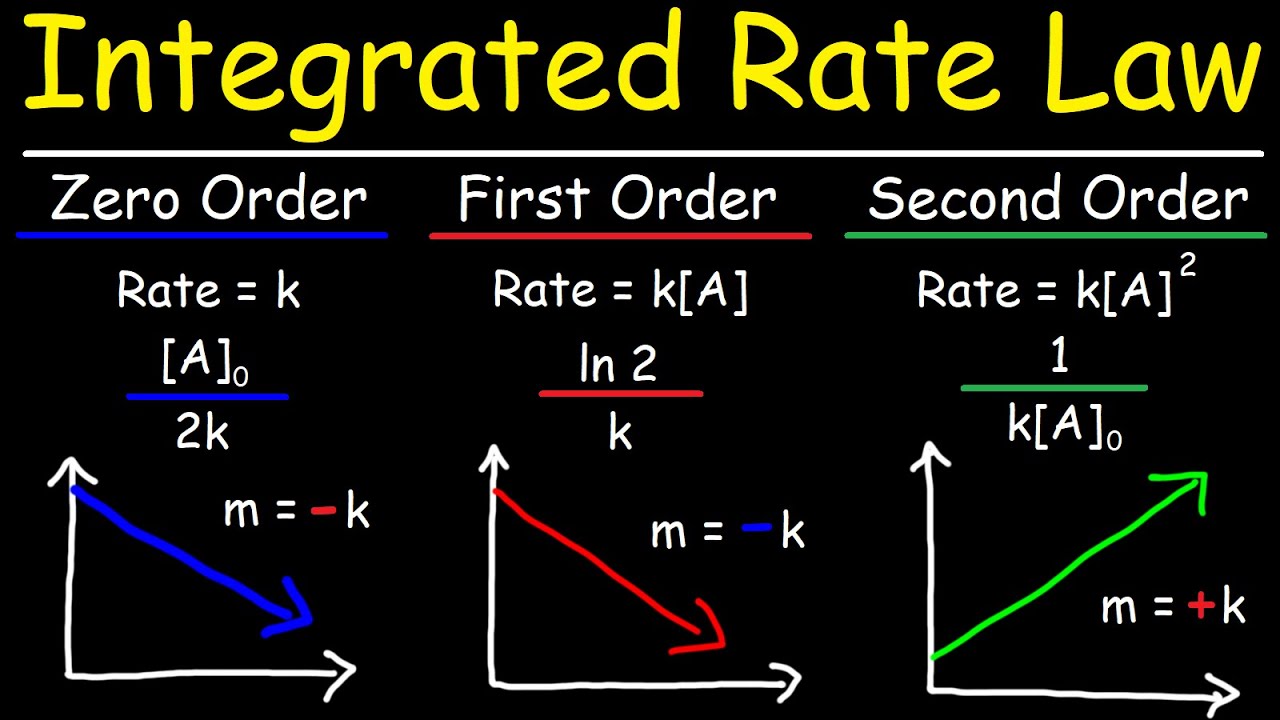
- 📉 Integrated rate laws provide different mathematical relationships for different reaction orders, which can be used to plot and analyze kinetic data.
- 📚 The units of the rate constant (k) are specific to the overall reaction order and must cancel out concentration units to result in molarity per second.
Q & A
What is the main focus of the script provided?
-The script focuses on the topic of kinetics, specifically discussing the study of reaction rates, how to measure them, and the factors that influence them.
How are reaction rates generally measured?
-Reaction rates are generally measured as an increase in the concentration of products per unit time or molarity per second, or as the change in concentration of reactants over change in time.
What does the term 'stoichiometry' refer to in the context of reaction rates?
-Stoichiometry refers to the balanced relationship between reactants and products in a chemical reaction, which dictates how the rates of change in concentration of different substances are related.
What is the difference between the instantaneous rate and the average rate?
-The instantaneous rate is the rate at a specific moment, calculated by the slope of the tangent line at a point on the concentration versus time plot. The average rate is calculated over a range of points, which is less precise than the instantaneous rate.
What is the significance of reaction order in understanding reaction rates?
-The reaction order with respect to a reactant indicates how the concentration of that reactant affects the rate of the reaction. It helps in determining the relationship between concentration changes and the rate of reaction.
How can the rate law be used to describe the kinetics of a reaction?
-The rate law represents the relationship between the rate of a reaction and the concentrations of the reactants, with each reactant raised to an exponent that reflects its reaction order. It includes a rate constant (k), which is determined experimentally.
What method is used to experimentally determine the order of a reaction with respect to each reactant?
-Initial rates data is used, where several trials of a reaction are run, varying the initial concentration of one reactant at a time, to observe the effect on the rate.
How can the rate constant be calculated from experimental data?
-The rate constant can be calculated by plugging in the rate and concentrations from any one of the trials into the rate law and solving for k.
What is the relationship between the units of the rate constant and the overall reaction order?
-The units of the rate constant are specific to the overall reaction order and must cancel out the concentration units to result in molarity per second, which is the unit for the rate.
What are the integrated rate laws, and how can they be used to discern the reaction order?
-Integrated rate laws are mathematical expressions that relate the concentration of reactants or products to time for different reaction orders. They can be plotted in a linear format (y = mx + b), and the resulting straight line can indicate the overall reaction order through graphical analysis.
Outlines
🔍 Introduction to Chemical Kinetics
Professor Dave introduces the concept of kinetics, which is the study of reaction rates or the speed at which chemical reactions occur. He explains that reaction rates can vary greatly, and this variability is the focus of kinetics. The rate of a reaction is typically measured by the increase in product concentration over time or by the decrease in reactant concentration. The relationship between the rate of change and the stoichiometry of the reaction is discussed, emphasizing that the rate of disappearance or appearance of substances in a reaction is proportional to their stoichiometric coefficients. Various methods for measuring reaction rates are mentioned, such as monitoring gas pressure or light absorbance, and the importance of plotting concentration versus time to calculate instantaneous rates is highlighted.
📚 Understanding Reaction Rates and Orders
This paragraph delves deeper into how reaction rates depend on the concentration of reactants and introduces the concept of reaction order. It explains how doubling the concentration of a reactant can affect the rate, indicating the reaction order with respect to that reactant. The overall reaction order is the sum of the individual orders of the reactants. The rate law is introduced, which is an equation that relates the rate of a reaction to the concentrations of the reactants, raised to powers that represent their respective orders. The importance of experimentally determining these orders and the role of the rate constant 'k' in connecting rate and concentration is emphasized. The paragraph also discusses how to determine the reaction order for each reactant using initial rates data and provides an example of how to calculate the rate constant and its units based on the overall reaction order.
🧪 Determining Reaction Orders and Rate Constants
The script continues with a practical approach to experimentally determine the order of a reaction with respect to each reactant by using initial rates data. It provides a step-by-step method to perform trials with varying initial concentrations of reactants to observe their impact on the reaction rate. An example is given where the reaction order with respect to NO and O2 is determined by analyzing the rate changes in different trials. The rate law for the reaction is deduced based on these observations, and it is noted that the reaction orders coinciding with stoichiometric coefficients is not a rule but a coincidence. The paragraph concludes with a discussion on calculating the rate constant from rate data and understanding its units in relation to the overall reaction order.
Mindmap
Keywords
💡Kinetics
💡Reaction Rates
💡Concentration
💡Stoichiometry
💡Instantaneous Rate
💡Reaction Order
💡Rate Law
💡Initial Rates Method
💡Rate Constant (k)
💡Integrated Rate Law
💡Spectrophotometer
Highlights
Kinetics is the study of reaction rates, explaining why some chemical reactions are faster than others.
Reaction rates are measured as the increase in product concentration per unit time or molarity per second.
The concept of 'change in concentration' is denoted by the Greek letter delta (Δ), with brackets indicating concentration.
Stoichiometry plays a role in reaction rates, with products appearing at rates proportional to their stoichiometric coefficients.
Reaction rates can be measured by observing changes in gas pressure or light absorbance in colored solutions.
Instantaneous rate is calculated by the slope of the tangent line to a point on the concentration-time plot.
The rate of a reaction depends on the concentration of one or more reactants, described by the reaction order.
Reaction order is determined experimentally by observing the effect of concentration changes on the rate.
The overall reaction order is the sum of the individual orders of the reactants involved.
Rate laws are expressed using the concentration of reactants raised to an exponent reflecting the reaction order.
The rate constant (k) is a proportionality constant between rate and concentration, determined experimentally.
Initial rates data is used to experimentally determine the order of a reaction with respect to each reactant.
The units of the rate constant are specific to the overall reaction order, ensuring the rate is expressed in molarity per second.
Integrated rate laws provide different mathematical relationships for different reaction orders, allowing for graphical analysis.
For zero-order reactions, a plot of concentration versus time yields a straight line.
For first-order reactions, a plot of the natural log of concentration versus time yields a straight line.
For second-order reactions, a plot of the inverse of concentration versus time yields a straight line.
The rate law and reaction orders can be discerned from the stoichiometry of the reaction, but this is not always the case.
Understanding the rate constant and its units can help deduce the overall reaction order.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: