Chemical Kinetics summary in 30 minutes
TLDRThis tutorial offers a comprehensive introduction to chemical kinetics, focusing on reaction rates and mechanisms. It explains how factors such as temperature, concentration, catalysts, surface area, and pressure influence reaction speeds. The video also delves into rate laws and reaction orders, illustrating how to determine the half-life of substances in zero, first, and second-order reactions. By using equations and experimental data, the tutorial aims to demystify chemical kinetics and empower viewers with the tools to predict and analyze reaction dynamics.
Takeaways
- 📚 Chemical kinetics involves studying the rate at which a chemical reaction progresses and the mechanisms behind these reactions.
- 🔍 Understanding reaction rates is crucial, which are influenced by factors such as the concentration of reactants and the rate constant (k).
- 🌡️ Temperature plays a significant role in reaction rates; higher temperatures generally increase the rate of reaction due to the collision theory and increased kinetic energy of particles.
- 🧪 Concentration of reactants affects the rate of reaction, with higher concentrations leading to more frequent collisions and thus faster reactions.
- 💡 Catalysts speed up reactions by lowering the activation energy required, without being consumed in the reaction.
- 📏 Surface area of solid reactants impacts the reaction rate, with greater surface areas leading to more collisions and faster reactions.
- 🌬️ Pressure affects the rate of reactions involving gaseous reactants, with higher pressures resulting in more frequent collisions.
- 📈 Determining reaction rates can be done by monitoring changes in concentration of either reactants or products over time.
- 📊 Rate laws are specific to each reaction and express the rate as a function of concentration, with orders of reactions (zero, first, second, etc.) being key to understanding these relationships.
- 🔍 Predicting the order of reactions can be done through experimental data or by analyzing the units of the rate constant.
- 🕰️ Half-life calculations for different orders of reactions are essential in understanding how long it takes for the concentration or mass of a substance to reduce by half.
Q & A
What is chemical kinetics?
-Chemical kinetics is the study of the rates at which chemical reactions progress and the mechanisms by which they occur.
What are the two main aspects that chemical kinetics deals with?
-Chemical kinetics primarily deals with the rate of chemical reactions and the reaction mechanisms, which explains the series of steps involved in a chemical reaction.
How is the reaction rate expressed mathematically?
-The reaction rate is expressed as the change in concentration of reactants or products per unit time, often denoted by the symbol 'r' and determined by the stoichiometric coefficients of the reactants.
What factors affect the rate constant in a chemical reaction?
-The rate constant is affected by factors such as temperature, concentration of reactants, presence of a catalyst, surface area of solid reactants, and pressure of gaseous reactants.
How does temperature influence the rate of a chemical reaction?
-As temperature increases, the kinetic energy of the particles also increases, leading to more frequent and energetic collisions, which in turn increases the rate of the chemical reaction.
What is the collision theory and its significance in chemical kinetics?
-The collision theory states that for a chemical reaction to occur, reactant molecules must collide with each other with sufficient energy, known as activation energy. It explains why higher temperatures typically lead to faster reaction rates.
What is a catalyst and how does it affect the rate of a chemical reaction?
-A catalyst is a substance that speeds up the rate of a chemical reaction by lowering the activation energy required for the reaction to occur. It is not consumed in the reaction itself.
How does the surface area of solid reactants impact the rate of a chemical reaction?
-An increase in the surface area of solid reactants leads to more frequent collisions with other reactants, thus increasing the rate of the chemical reaction.
What is the relationship between the pressure and the rate of reaction for gaseous reactants?
-Higher pressure leads to a greater number of gas molecules in a given volume, resulting in more frequent collisions and thus an increased rate of reaction.
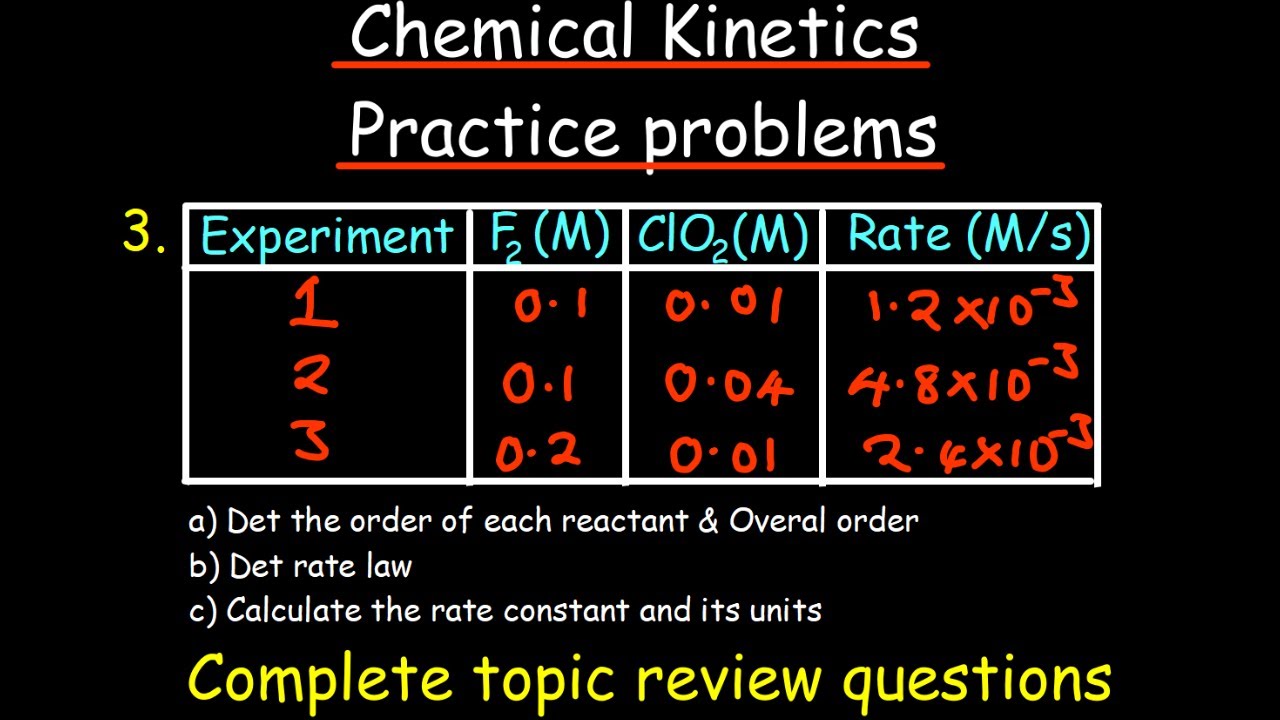
How can the order of a reaction be determined from experimental data?
-The order of a reaction can be determined by analyzing the relationship between the reaction rate and the concentration of the reactants using graphical methods or by examining the units of the rate constant in relation to the reaction.
What is the half-life of a reaction and how is it calculated for zero-order reactions?
-The half-life of a reaction is the time taken for the concentration or mass of a substance to reduce by half. For zero-order reactions, it can be calculated using the formula t1/2 = (2k)^(-1), where k is the rate constant.
What are the half-life formulas for first and second-order reactions?
-For first-order reactions, the half-life formula is t1/2 = ln(2) / k, and for second-order reactions, it is t1/2 = 1 / (a_initial * k), where a_initial is the initial concentration of the reactant and k is the rate constant.
Outlines
📚 Introduction to Chemical Kinetics
This paragraph introduces the concept of chemical kinetics, aiming to simplify its understanding. It defines chemical kinetics as the study of the rate at which chemical reactions progress and touches on reaction mechanisms, which explain the steps involved in a chemical reaction. The paragraph emphasizes the importance of reaction rates and the factors affecting them, such as the rate constant (k), and introduces the concept of activation energy and collision theory, linking temperature and reaction rate.
🌡️ Factors Affecting Reaction Rates
This section delves into the various factors that influence the rate of chemical reactions. It discusses how temperature, concentration, catalysts, surface area of solid reactants, and pressure of gaseous reactants play a role in the speed of reactions. The paragraph explains how increased temperature can lead to faster reactions due to higher kinetic energy, and how concentration and surface area can increase the likelihood of successful collisions between reactant particles. The role of catalysts in lowering activation energy and thus speeding up reactions is also highlighted.
📈 Determining Reaction Rates
The paragraph explains how to determine the reaction rate by monitoring changes in the concentration of reactants or products. It introduces the concept of stoichiometric coefficients and how they are used in rate equations. The paragraph provides examples of how to express reaction rates for different reactants and products, emphasizing the use of negative signs to ensure that the rate remains a positive value. It also explains the rationale behind using negative signs in calculations to maintain consistency in rate determination.
📝 Rate Laws and Reaction Orders
This section introduces rate laws and the concept of reaction orders. It explains how each reaction has its own rate law, which is a function of the concentration of reactants or products. The paragraph discusses how rate laws are experimentally determined and how they can be expressed in terms of reaction orders. It also introduces the idea of zero, first, and second-order reactions, providing equations for each and explaining how to predict the order of a reaction from experimental data.
🕒 Half-Life of Reaction Rates
The paragraph focuses on the concept of half-life in the context of reaction rates. It explains how to calculate the half-life for zero, first, and second-order reactions, providing formulas for each. The explanation includes the rationale behind the formulas and how they are derived from the rate equations. The paragraph emphasizes the importance of understanding half-life in predicting the time it takes for a reaction to proceed to a certain extent.
📊 Predicting Reaction Orders from Data
This section discusses methods for predicting the order of a reaction using experimental data. It explains how to plot concentration versus time graphs for zero, first, and second-order reactions and how to interpret these graphs to determine the reaction order. The paragraph highlights the importance of graphing data to visually identify whether the reaction follows a straight line, which would indicate the order of the reaction.
🔄 Conclusion and Future Tutorials
In conclusion, the paragraph recaps the key points covered in the video, including the basics of chemical kinetics, factors affecting reaction rates, determining reaction rates, rate laws, reaction orders, and half-life calculations. It also teases upcoming tutorials that will cover more advanced topics in chemical kinetics, such as the Arrhenius equation and other related concepts, encouraging viewers to stay tuned for further learning.
Mindmap
Keywords
💡Chemical Kinetics
💡Reaction Rate
💡Reaction Mechanisms
💡Rate Constant (k)
💡Temperature
💡Concentration
💡Catalyst
💡Activation Energy
💡Surface Area
💡Pressure
💡Stoichiometric Coefficients
Highlights
Chemical kinetics is the study of how fast a chemical reaction progresses.
Reaction mechanisms explain the series of steps involved in a chemical reaction.
Reaction rates are used to understand the speed at which a reaction occurs.
The rate constant (k) is a factor that affects the speed of a reaction; a higher k means a faster reaction.
Temperature is a key factor affecting reaction rates; higher temperatures generally increase reaction speeds.
The collision theory explains the necessity of collisions with sufficient energy for chemical reactions to occur.
Activation energy is the minimum energy required for a reaction to proceed.
Concentration of reactants is another factor that influences the rate of a chemical reaction.
Catalysts speed up reactions by lowering the activation energy without being consumed in the reaction.
Surface area of solid reactants affects the rate of reaction; greater surface area leads to faster reactions.
Pressure affects the rate of reactions involving gaseous reactants; higher pressure leads to more frequent collisions.
Reaction rates can be determined by monitoring changes in concentration of reactants or products.
Rate laws express the rate of a reaction as a function of the concentration of reactants.
Zero order reactions have a rate that is independent of the concentration of reactants.
First order reactions depend on the natural logarithm of the concentration of reactants.
Second order reactions involve the concentration of reactants squared in their rate equations.
Half-life calculations are crucial for understanding the time taken for the concentration or mass of a substance to reduce by half.
The order of a reaction can be determined from experimental data and is important for predicting reaction behavior.
Half-life formulas for zero, first, and second order reactions are derived to understand the time dynamics of reactions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: