30. Kinetics: Rate Laws
TLDRThe lecture delves into the realm of chemical kinetics, emphasizing its interplay with thermodynamics. It explores the factors influencing reaction rates, such as temperature and catalysts, and introduces the concept of stability and spontaneity in reactions. Through the oscillating clock reaction, the instructor illustrates the dynamics of chemical equilibrium and the impact of temperature on reaction rates. The lecture also covers the measurement of average and instantaneous rates, the formulation of rate expressions and laws, and the significance of reaction orders. It concludes with an introduction to integrated rate laws and half-life, exemplified by radioactive decay, highlighting the experimental determination of rate constants.
Takeaways
- 📚 The lecture introduces the concept of kinetics, emphasizing its importance alongside thermodynamics in understanding chemical reactions.
- 🔍 The difference between stability/instability and labile/inert is explained, with the former relating to thermodynamics (ΔG) and the latter to kinetics (rate of reaction).
- 🌡 The impact of temperature on reaction rates is discussed, highlighting how it can speed up or slow down chemical reactions.
- 🔄 The concept of chemical kinetics involves measuring how concentrations change over time, which is crucial for understanding reaction rates.
- 🔬 Factors affecting reaction rates are explored, including temperature, catalysts, pressure, the nature of the material (solid, liquid, gas), and the reaction mechanism.
- 🎡 The 'oscillating clock' reaction is presented as an example of a complex chemical reaction involving multiple steps, demonstrating concepts of thermodynamics, kinetics, and chemical equilibrium.
- 📉 The demonstration of the oscillating clock reaction visually shows how temperature affects reaction rates, with a colder reaction proceeding much slower than a warmer one.
- ⏱ The distinction between average and instantaneous rates is made, with the former being dependent on the time interval chosen and the latter representing the rate at a specific moment.
- 📘 The process of deriving and using integrated rate laws for first-order reactions is explained, allowing for the calculation of rate constants and understanding of reaction progress over time.
- 💊 The half-life of a first-order reaction is introduced, noting that it is a constant time for a substance to decay to half its initial amount, independent of the initial concentration.
- ☢️ Radioactive decay is cited as a real-world example of a first-order process, illustrating the application of kinetic principles beyond the classroom.
Q & A
What is the main topic of the lecture?
-The main topic of the lecture is chemical kinetics, which includes discussions on thermodynamics, reaction rates, and factors affecting the rates of chemical reactions.
Why is it important to consider both kinetics and thermodynamics when studying chemical reactions?
-Kinetics and thermodynamics are important to consider together because thermodynamics tells us if a reaction will proceed spontaneously, while kinetics tells us how fast the reaction will occur. Understanding both helps in predicting the feasibility and speed of a reaction.
What does the term 'stable/unstable' refer to in the context of thermodynamics and kinetics?
-In the context of thermodynamics and kinetics, 'stable/unstable' refers to the spontaneity of a reaction, which is related to the change in Gibbs free energy (delta G), not the rate of the reaction.
What is the difference between 'labile' and 'stable' as mentioned in the script?
-'Labile' refers to a substance that is prone to change or react easily, indicating a fast reaction rate. 'Stable', on the other hand, means that the substance is not reactive or inert, which is related to a negative delta G of formation.
How does the rate of a chemical reaction relate to the concentration of reactants?
-The rate of a chemical reaction can be affected by the concentration of reactants. Higher concentrations generally lead to more frequent collisions between reactant molecules, potentially increasing the reaction rate.
What is an example of a chemical reaction that involves multiple steps and changes in color?
-The oscillating clock reaction is an example of a chemical reaction that involves multiple steps and changes in color. It includes steps where the solution changes from clear to amber and then to a blue complex.
Why is hydrogen peroxide used in the oscillating clock reaction?
-Hydrogen peroxide is used in the oscillating clock reaction because it is a good oxidizing agent due to its large positive standard reduction potential, which makes the oxidation process spontaneous.
How does temperature affect the rate of the oscillating clock reaction?
-Temperature has a significant effect on the rate of the oscillating clock reaction. Higher temperatures increase the kinetic energy of the molecules, leading to a faster reaction rate. Conversely, lower temperatures slow down the reaction rate.
What is the difference between average rate and instantaneous rate in chemical kinetics?
-The average rate is calculated over a specific time interval and represents the mean change in concentration over that period. The instantaneous rate, on the other hand, is the rate at a specific moment in time and can be found by taking the limit as the time interval approaches zero.
What is the significance of the integrated rate law for a first-order reaction?
-The integrated rate law for a first-order reaction allows for the direct calculation of the concentration of a reactant at any time, based on its initial concentration and the reaction's rate constant. It also enables the determination of the rate constant by plotting the natural logarithm of the reactant concentration against time, which should yield a straight line.
How does the half-life of a first-order reaction relate to the rate constant?
-The half-life of a first-order reaction is the time it takes for the concentration of a reactant to decrease to half of its initial value. It is determined solely by the rate constant (k) and is independent of the initial concentration of the reactant.
Outlines
📚 Introduction to Kinetics and Thermodynamics
The script begins with an introduction to the MIT OpenCourseWare project and its mission to provide free educational resources. The lecturer, Catherine Drennan, then dives into the topic of kinetics, explaining its importance alongside thermodynamics in understanding chemical reactions. She emphasizes the distinction between stability, which is a thermodynamic concept related to delta G, and reaction rates, which are governed by kinetics. The lecture aims to explore these concepts throughout the semester, starting with a review of thermodynamics and kinetics, and their relevance to the spontaneity and speed of reactions.
🔍 Factors Influencing Reaction Rates
This paragraph explores the factors that affect the rate of chemical reactions. Temperature and catalysts are highlighted as key influencers, with pressure and the nature of the material (gas, solid, etc.) also playing a role. The complexity of reaction mechanisms is introduced, including the possibility of multiple steps and phase changes. The lecturer also touches on the concentration of reactants and how it relates to pressure, setting the stage for a deeper dive into these topics in subsequent lectures.
🧪 The Oscillating Clock Reaction Demonstration
The lecturer presents the 'oscillating clock reaction' as an example of a chemical reaction that incorporates various concepts from the course, such as thermodynamics, chemical equilibrium, kinetics, and acid-base chemistry. The reaction involves multiple steps and changes in color due to the oxidation states of the materials involved. The lecturer explains the process of the reaction, which includes the formation of an amber-colored solution that turns blue due to the formation of a complex, and how the reaction oscillates between these states.
📉 Oxidation-Reduction Reactions and Hydrogen Peroxide
The script delves into the specifics of oxidation-reduction reactions within the context of the oscillating clock reaction. The audience is guided through a chart-filling exercise to understand the changes in oxidation states of various elements involved in the reaction. Hydrogen peroxide is highlighted as a key component due to its tendency to be reduced, making it an effective oxidizing agent. The讲师 demonstrates the reaction's sensitivity to temperature, showing how a lower temperature can significantly slow the reaction.
📊 Measuring Reaction Rates: Average vs. Instantaneous
The lecturer introduces the concepts of average and instantaneous reaction rates, using a hypothetical chemical reaction as an example. The process of measuring the change in concentration of reactants or products over time is explained, and how these measurements can be used to calculate the average rate. The instantaneous rate is also discussed, which is the rate at a specific time point, and is represented by the slope of the tangent to the concentration-time curve at that point.
🔍 Rate Expressions and Rate Laws
The difference between rate expressions and rate laws is clarified. Rate expressions are based on the stoichiometry of the reaction and assume no intermediates are formed. In contrast, rate laws relate the rate of a reaction to the concentrations of reactants, with a proportionality constant known as the rate constant. The讲师 emphasizes that rate laws are determined experimentally and are not solely based on stoichiometry.
📚 Understanding Reaction Orders and Their Effects
This section discusses the concept of reaction orders, explaining how the order of a reaction in a particular substance affects the rate of the reaction. The讲师 uses a table to illustrate different orders of reactions, from first-order to zero-order, and how doubling the concentration of a reactant affects the rate differently depending on the order. The importance of experimental determination of reaction orders is stressed.
📉 Integrated Rate Laws and Radioactive Decay
The lecturer introduces integrated rate laws as a method to measure changes in concentration over time, which can be particularly useful for obtaining rate constants. The focus is on first-order reactions, where the integrated rate law is derived and presented in the form of a straight line equation. The concept of half-life is also introduced, showing that it is dependent on the rate constant and not on the initial concentration. Radioactive decay is given as an example of a first-order process.
📈 Plotting First-Order Reactions and Determining Rate Constants
The script concludes with a practical application of first-order integrated rate laws, demonstrating how to plot the natural logarithm of the concentration of a reactant against time to obtain a straight line. The y-intercept and slope of this line provide the original concentration and the negative of the rate constant, respectively. This method allows for the experimental determination of rate constants for various materials.
Mindmap
Keywords
💡Enzymes
💡Kinetics
💡Thermodynamics
💡Stability
💡Rate
💡Catalysts
💡Oscillating Reaction
💡Oxidation-Reduction Reactions
💡Integrated Rate Law
💡Half-Life
💡Radioactive Decay
Highlights
Enzymes are catalysts, and the discussion shifts to kinetics, the study of reaction rates, which is the last unit of the semester.
Kinetics and thermodynamics are interrelated, with the former focusing on the speed of reactions and the latter on their spontaneity.
The concept of stability in thermodynamics is distinguished from the rate of reactions in kinetics, with delta G being a key indicator.
An example of kinetics in everyday life is given through the average rate of a Ferris wheel's rotation, illustrating the importance of reaction rates.
Factors affecting chemical reaction rates, such as temperature, catalysts, and pressure, are discussed.
The oscillating clock reaction is introduced as a complex chemical reaction involving multiple steps and concepts from previous lessons.
The oscillating clock reaction demonstrates the interplay between chemical equilibrium, thermodynamics, and kinetics.
The importance of understanding the oxidation-reduction reactions and color changes in the oscillating clock reaction is emphasized.
A clicker question engages the audience to think about the role of hydrogen peroxide in the oscillating clock reaction.
The demonstration of the oscillating clock reaction visually shows the color changes and the effect of temperature on reaction rates.
The difference between average and instantaneous rates in chemical kinetics is explained, with examples provided.
The method for calculating the instantaneous rate at a specific time point is discussed, highlighting its importance in understanding reaction dynamics.
Rate expressions and rate laws are differentiated, with the latter including a rate constant and being determined experimentally.
The concept of reaction orders (first, second, etc.) is introduced, explaining how they affect the rate of reactions when concentrations change.
The overall order of a reaction is defined as the sum of the exponents in the rate law, which is crucial for understanding reaction kinetics.
Integrated rate laws are introduced as an alternative to measuring initial rates, especially useful for detecting small changes in concentration over time.
The derivation and application of the integrated first-order rate law are explained, showing how to calculate remaining concentrations over time.
Half-life is defined for first-order reactions, demonstrating that it is independent of concentration and depends solely on the rate constant.
The relationship between the rate constant, half-life, and the material's properties in first-order reactions is clarified.
Radioactive decay is presented as a real-world example of a first-order process, linking theoretical concepts to practical applications.
Transcripts
Browse More Related Video

AP Chem Unit 5 Review - Kinetics in 10 Minutes!
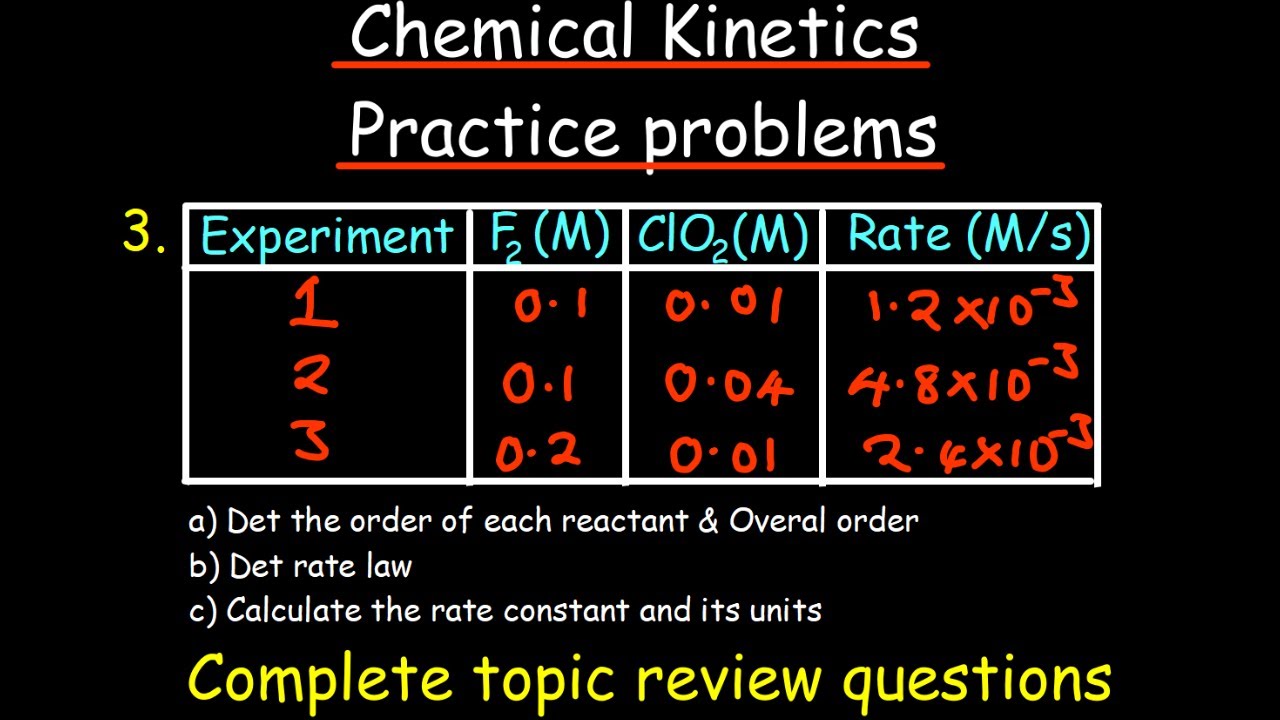
Chemical Kinetics practice problems - complete review
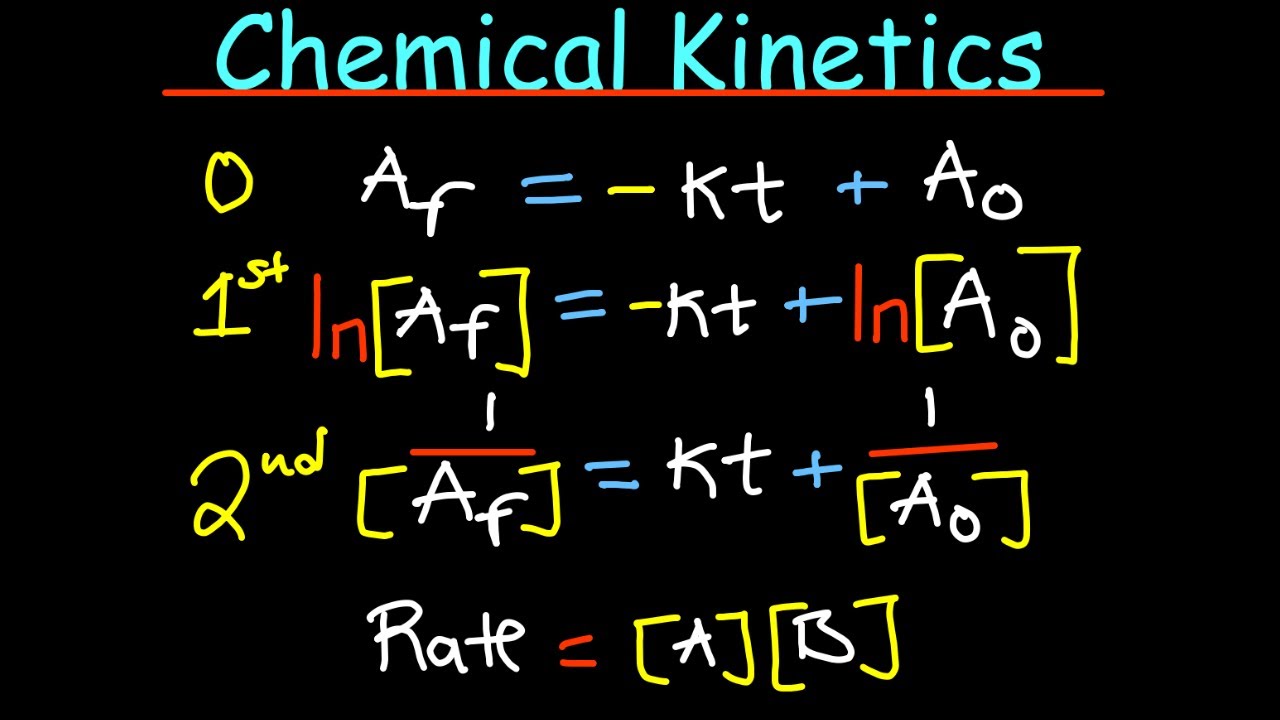
Chemical Kinetics summary in 30 minutes

Reaction Kinetics Concepts

Kinetics: Initial Rates and Integrated Rate Laws

2022 Live Review 2 | AP Chemistry | Kinetics Multiple-Choice and Free-Response Questions
5.0 / 5 (0 votes)
Thanks for rating: