AP Chem - Full kinetics review guide
TLDRThis AP Chemistry lesson delves into the fundamental concept of kinetics, essential for understanding how molecules interact and transform. It covers reaction rates, factors influencing them like concentration, activation energy, and temperature, and introduces the rate laws and their experimental determination. The video also explains half-life calculations, pseudo first-order reactions, and graphical analysis for determining reaction order. Additionally, it touches on reaction mechanisms, the collision model, activation energy, and the role of catalysts in facilitating reactions, aiming to prepare students for their kinetics exam.
Takeaways
- 🔬 Chemical kinetics is the study of reaction rates and the mechanisms of chemical reactions.
- 📈 The rate of a reaction is analogous to velocity, measuring the change in concentration over time.
- 🌡 Factors such as concentration, activation energy, temperature, and surface area of solids affect reaction rates.
- 📚 The Arrhenius equation shows the relationship between the rate constant and activation energy as well as temperature.
- 🔢 The rate law is an equation that relates the rate of a reaction to the concentration of reactants and a rate constant.
- 🧬 Integrated rate laws express how concentration changes over time for zero, first, and second order reactions.
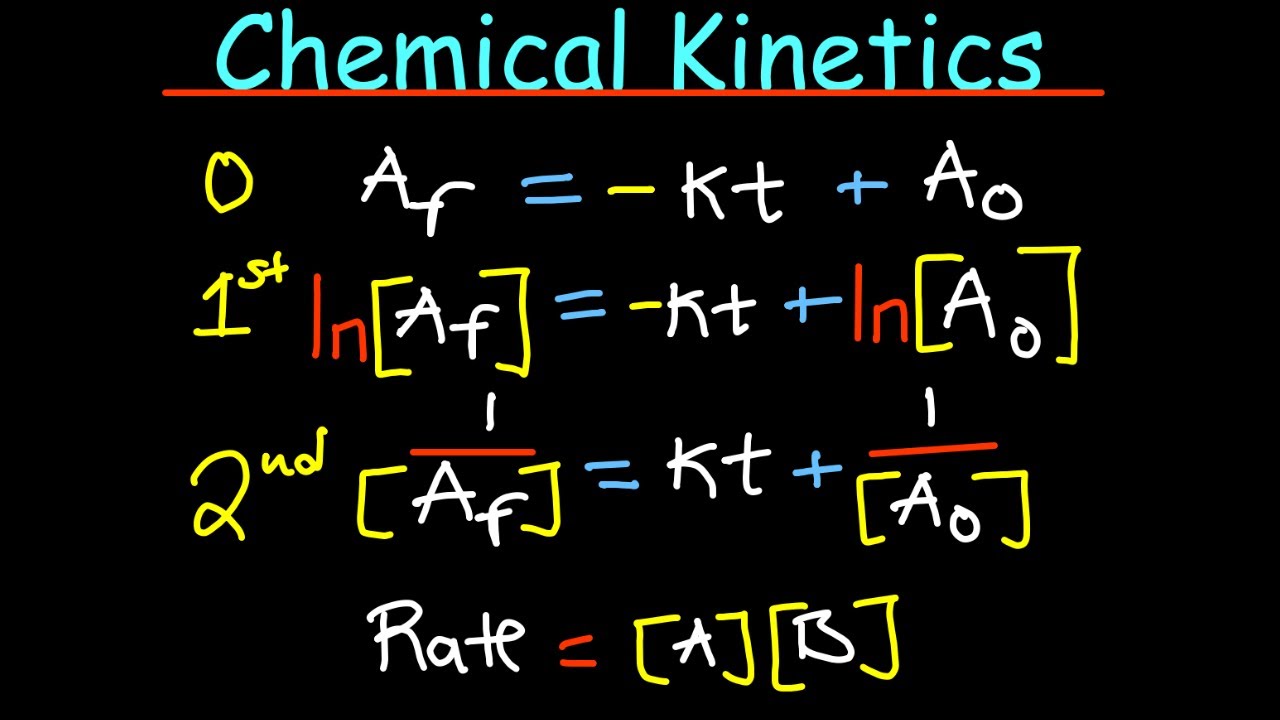
- ⏱ The half-life of a reaction can be derived from its rate law, indicating the time for the concentration to decrease by half.
- 📉 Pseudo first-order reactions occur when one reactant's concentration is significantly smaller than another, simplifying the rate law.
- 📊 Graphs of concentration over time can help determine the order of a reaction and its rate constant.
- 💡 Reaction mechanisms involve a series of elementary reactions, with the slowest step determining the overall rate.
- 🛠 Collision model explains how molecules must collide with the correct orientation and sufficient energy to react, forming activated complexes.
Q & A
What is the main topic of the video script?
-The main topic of the video script is chemical kinetics, which is essential for understanding the rates of chemical reactions and the mechanisms by which they occur.
What is the definition of chemical kinetics?
-Chemical kinetics refers to the study of the rates of chemical reactions and the mechanisms by which these reactions occur.
How is the rate of a chemical reaction defined?
-The rate of a chemical reaction is defined as the change in concentration of a product or reactant over a unit of time.
What factors affect the reaction rates?
-Reaction rates are affected by factors such as the concentration of reactants, activation energy, temperature, and the surface area of solids.
What is the Arrhenius equation and its significance?
-The Arrhenius equation is used to express the temperature dependence of reaction rates. It shows that the rate constant (K) increases when activation energy decreases and when temperature increases.
What is the role of the rate constant (k) in chemical kinetics?
-The rate constant (k) is an experimentally determined coefficient that is a proportionality constant in the relationship between the rate of a reaction and the concentration of the reactants.
What are rate laws and why are they important?
-Rate laws are equations that relate the rate of a chemical reaction to the concentration of the reactants, the rate constant, and time. They are important for understanding how the rate of a reaction is influenced by the concentrations of the reactants.
What is the difference between the differential rate law and the integrated rate law?
-The differential rate law shows how the rate of a reaction depends on the concentration of the reactants, while the integrated rate law expresses how the concentration of a reactant changes over time.
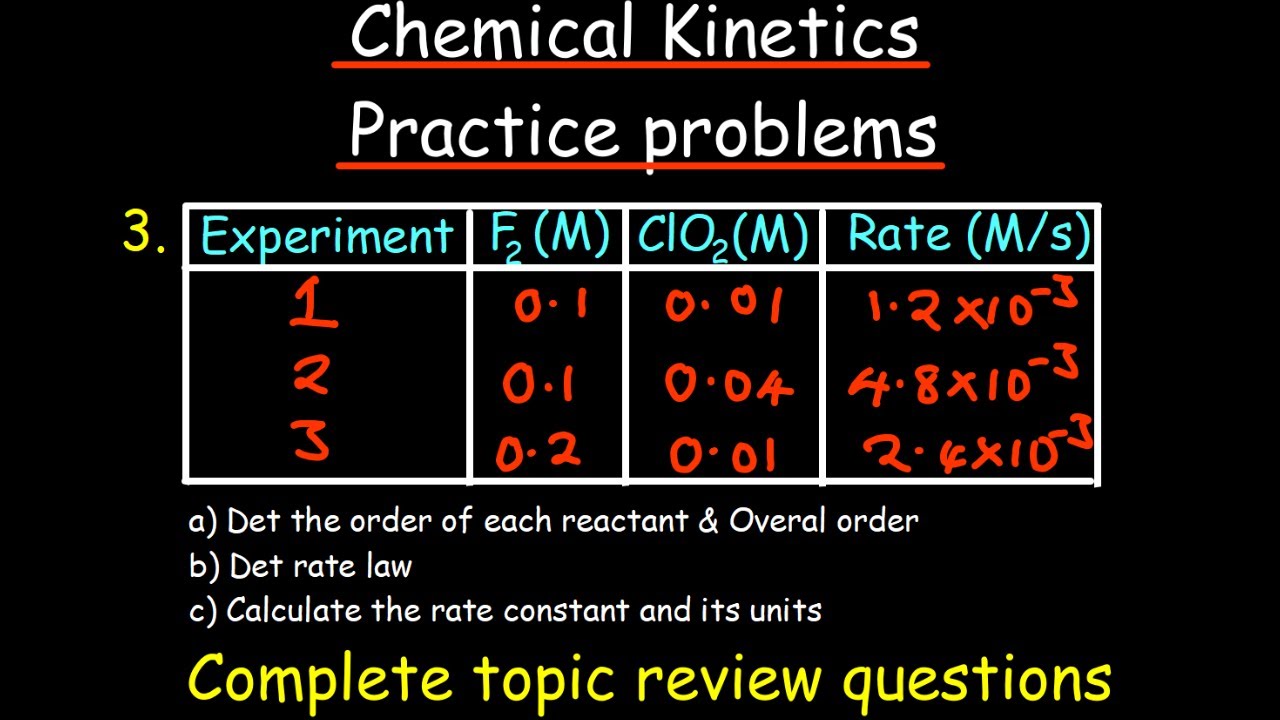
How can you determine the order of a reaction experimentally?
-The order of a reaction can be determined experimentally by finding the exponents of the reactants from the rate law, using the concentrations and rates of the reaction at different points.
What is the significance of half-life in chemical kinetics?
-The half-life of a reaction is the time it takes for the concentration of a reactant to decrease by half. It is derived from the rate law and provides insight into the time scale of a reaction.
What is a catalyst and how does it affect chemical reactions?
-A catalyst is a substance that increases the rate of a reaction without being consumed in the process. It works by lowering the activation energy and facilitating the correct orientation of reactant molecules, thus speeding up the reaction.
What is the collision model and its role in explaining chemical reactions?
-The collision model attempts to explain how reactions occur by stating that molecules must collide with each other to react. It considers factors such as activation energy and the correct orientation of molecules during collisions to determine whether a reaction will proceed.
How can activation energy be determined using the Arrhenius equation?
-Activation energy can be determined using the Arrhenius equation by plotting the natural logarithm of the rate constant (K) against the inverse of the temperature (1/T) and finding the slope of the resulting line, which is equal to -activation energy divided by the gas constant (R).
What is the significance of a potential energy diagram in understanding chemical reactions?
-A potential energy diagram shows the change in energy as a reaction progresses, including the energy of reactants, the energy of the activated complex, and the energy of the products. It helps visualize the activation energy and the overall energy change of the reaction, indicating whether the reaction is exothermic or endothermic.
Outlines
🔬 Chemistry Kinetics and Reaction Rates
This paragraph introduces the fundamental concept of chemical kinetics, which is pivotal for understanding how chemical reactions occur and the factors influencing their rates. It explains that kinetics is the study of reaction rates and mechanisms, emphasizing the importance of how reactants interact to form products. The rate of a reaction is defined as the change in concentration of a product or reactant over time, drawing an analogy to velocity in terms of change in distance over time. The paragraph also touches on reaction mechanisms and the factors that affect reaction rates, such as concentration, activation energy, temperature, and surface area of solids. The Arrhenius equation is introduced to illustrate how the rate constant K is influenced by activation energy and temperature.
📚 Understanding Rate Laws and Half-Life
This section delves deeper into the specifics of rate laws, which are mathematical expressions that relate the rate of a chemical reaction to the concentrations of the reactants. It explains the differential rate law and the integrated rate law, highlighting how they differ in their approach to expressing the relationship between rate, concentration, and time. The paragraph also discusses how to experimentally determine the rate law by analyzing the concentrations and rates at different time points. The concept of half-life is introduced, explaining how it can be derived from the rate law and how it varies depending on the order of the reaction. The paragraph concludes with a discussion on pseudo first-order reactions, which occur when one reactant's concentration is significantly smaller than another, allowing for simplification of the rate law.
📉 Graphical Analysis and Reaction Mechanisms
This paragraph focuses on the graphical representation of reaction rates over time for different orders of reactions, providing a method to determine the order of a reaction experimentally by analyzing the correlation coefficient of the graph. It also discusses the complexities of reaction mechanisms, explaining that not all chemical reactions occur in a single step and introducing the concept of elementary reactions and intermediates. The rate-determining step is identified as the slowest step in a series of reactions that dictates the overall rate of the reaction. The paragraph further explores how to express the rate law in terms of non-intermediate species when intermediates are involved in the slow step. The Collision model is introduced to explain the physical process of how reactions occur through molecular collisions, with activation energy and molecular orientation playing crucial roles in the reaction process.
🌡 Activation Energy and Catalysts in Reactions
The final paragraph discusses the determination of activation energy, which is the minimum energy required for a reaction to proceed, using the Arrhenius equation. It outlines two methods for determining activation energy: graphical analysis and a direct calculation using rate constants from two different temperatures. The paragraph also introduces potential energy diagrams to visualize the energy changes during a reaction, with the activated complex representing the high-energy transition state. Catalysts are highlighted as substances that can increase the rate of a reaction by lowering the activation energy and facilitating correct molecular orientation, without being consumed in the process.
📢 Conclusion and Engagement Invitation
In the concluding remarks, the script invites viewers to share their thoughts on the video, particularly their favorite parts of the kinetics lesson. It encourages engagement and feedback, aiming to create a dialogue with the audience about their experience and understanding of the topic.
Mindmap
Keywords
💡Chemical Kinetics
💡Reaction Rate
💡Activation Energy
💡Temperature
💡Surface Area
💡Arrhenius Equation
💡Rate Law
💡Half-life
💡Pseudo First-Order Reaction
💡Reaction Mechanism
💡Collision Model
💡Catalysts
Highlights
Chemical kinetics is essential for understanding how molecules interact and transform into other substances.
Kinetics focuses on the rates of chemical reactions and the mechanisms behind them.
Reaction rate is analogous to velocity, measuring the change in concentration over time.
The rate of a reaction is directly proportional to the concentration of reactants.
Activation energy is a threshold that must be overcome for a reaction to proceed.
Temperature and surface area are key factors influencing reaction rates.
The Arrhenius equation relates the rate constant to activation energy and temperature.
The order of a reaction is the sum of the exponents of the species affecting the rate.
Rate laws connect concentration, time, the rate constant, and the rate itself.
Differential and integrated rate laws are used to understand how rate depends on concentration and time, respectively.
Experimental data can be used to determine the exponents in a rate law.
Half-life is a measure of time for the concentration of a reactant to decrease by half.
Half-life formulas vary depending on the order of the reaction.
Pseudo first-order reactions occur when the concentration of one reactant is significantly smaller than another.
Reaction order can be determined experimentally using graphical methods and correlation coefficients.
Reaction mechanisms involve a series of elementary reactions, with the slowest step determining the overall rate.
The collision model explains how molecular collisions and activation energy influence reaction rates.
Activation energy can be determined graphically or through equations using rate constants and temperatures.
Potential energy diagrams illustrate the energy changes during a reaction, including activation energy.
Catalysts increase reaction rates by lowering activation energy and facilitating correct molecular orientation.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: