Ideal gas equation example 1 | Chemistry | Khan Academy
TLDRThis educational video script offers an in-depth exploration of the ideal gas equation, PV = nRT, through practical application. It explains the relationship between pressure, volume, the number of molecules, and temperature in an ideal gas. The script guides viewers through a problem involving a two-liter balloon of hydrogen gas at 30°C and 2 atmospheres of pressure, demonstrating how to calculate the number of moles of hydrogen present. It emphasizes the importance of using the correct proportionality constant and converting temperatures to Kelvin for accurate calculations. The video also touches on converting moles to grams and the significance of unit consistency in gas law problems.
Takeaways
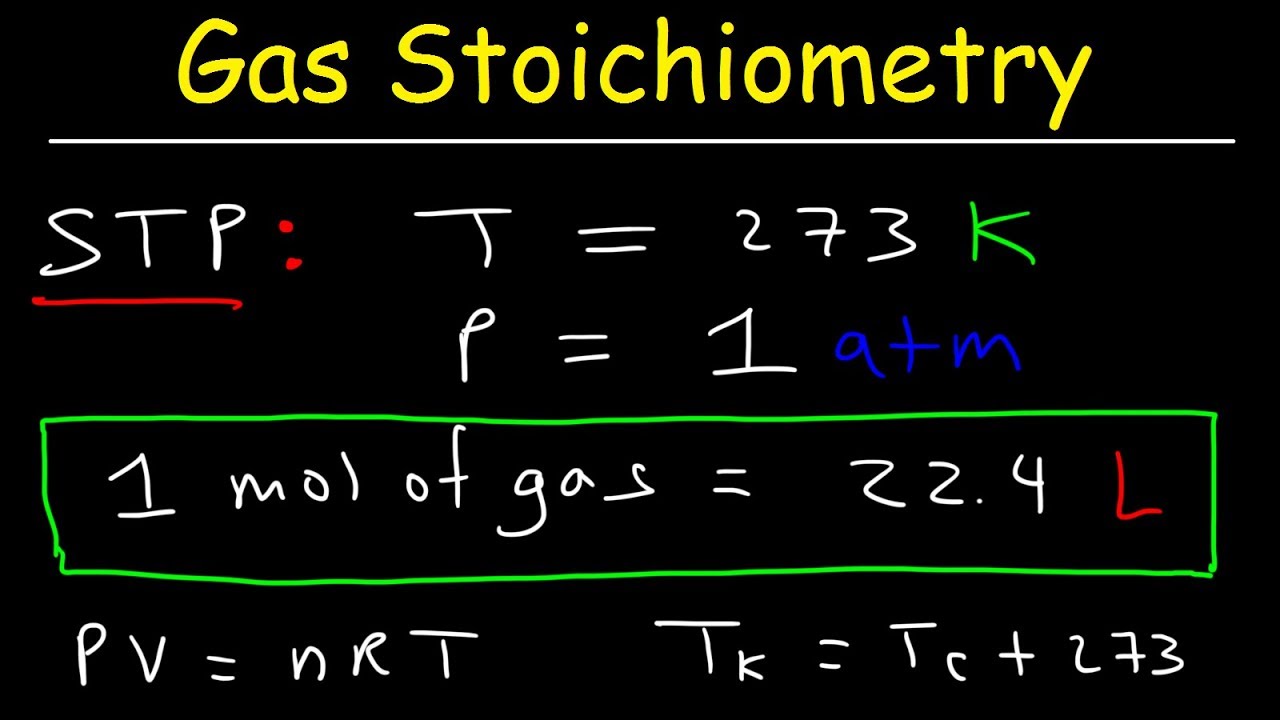
- 📚 The ideal gas equation is P × V = n × R × T, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin.
- 🔍 Understanding the relationship between pressure, volume, number of particles, and temperature is crucial for solving gas law problems.
- 🎈 The example given involves a 2-liter balloon filled with hydrogen gas at 30°C and 2 atmospheres of pressure.
- 🌡 Always convert Celsius temperatures to Kelvin when using the ideal gas equation, by adding 273 to the Celsius value.
- 📐 The Kelvin scale is an absolute temperature scale where 0 K represents absolute zero, the lowest possible temperature.
- ⚖️ The gas constant R used in calculations depends on the units of pressure and volume; for atmospheres and liters, R = 0.082 L·atm/(mol·K).
- 🔢 When performing calculations with the ideal gas equation, ensure that units cancel out appropriately to solve for the desired quantity, such as moles.
- 🧐 Dimensional analysis is a key technique in solving physics problems, treating units as algebraic quantities to ensure the equation balances.
- 📉 The calculation in the script shows that for the given conditions, there are approximately 0.16 moles of H₂ in the balloon.
- 🔎 Knowing the number of moles allows for further calculations, such as determining the number of molecules using Avogadro's number (approximately 6.02 × 10²³ molecules/mole).
- 📌 The mass of hydrogen gas can be found by multiplying the number of moles by the molar mass; for H₂, it's 2 grams per mole, resulting in 0.32 grams for 0.16 moles.
- 📚 The importance of using correct units and constants in gas law calculations is emphasized, as incorrect units can lead to erroneous results.
Q & A
What is the ideal gas equation?
-The ideal gas equation is PV = nRT, where P is the pressure, V is the volume, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature in Kelvin.
Why is it necessary to convert Celsius to Kelvin when using the ideal gas equation?
-Celsius is a relative temperature scale based on the freezing and boiling points of water, whereas Kelvin is an absolute scale starting at absolute zero. Kelvin is used in the ideal gas equation because it provides a more accurate representation of the energy state of the molecules.
How do you convert Celsius to Kelvin?
-To convert Celsius to Kelvin, add 273.15 to the Celsius temperature. For example, 30 degrees Celsius is equivalent to 303.15 Kelvin.
What is the significance of the proportionality constant R in the ideal gas equation?
-The proportionality constant R in the ideal gas equation is used to relate the pressure, volume, and temperature of a gas to the number of moles. Its value depends on the units used for pressure, volume, and temperature.
What is the value of R when using atmospheres and liters?
-When using atmospheres for pressure and liters for volume, the value of R is 0.082 liter atmospheres per mole Kelvin.
What is the relationship between the pressure and volume of an ideal gas?
-For an ideal gas, the pressure is inversely proportional to the volume when the number of moles and temperature are held constant. This means that as the volume increases, the pressure decreases, and vice versa.
How does the number of particles in a gas relate to its pressure and temperature?
-The pressure of an ideal gas is directly proportional to the number of particles and the temperature when the volume is held constant. More particles and higher temperature result in greater pressure.
What is a diatomic molecule, and how does it relate to hydrogen gas?
-A diatomic molecule is a molecule composed of two atoms, bonded together. Hydrogen gas (H2) is an example of a diatomic molecule, as each molecule consists of two hydrogen atoms.
How many moles of hydrogen gas are in a 2-liter balloon at 30 degrees Celsius and 2 atmospheres of pressure?
-Using the ideal gas equation with the given conditions, the number of moles of hydrogen gas can be calculated to be approximately 0.16 moles.
What is the mass of the hydrogen gas in the balloon from the previous question?
-Since one mole of H2 has a mass of 2 grams (because hydrogen is diatomic and each hydrogen atom has an atomic mass unit of 1), 0.16 moles of hydrogen gas would have a mass of 0.32 grams.
Why is it important to consider units when solving problems with the ideal gas equation?
-Units are crucial in the ideal gas equation because they must be consistent and correctly applied to ensure the mathematical validity of the solution. Different units for pressure, volume, or temperature require different values of R.
Outlines
📚 Ideal Gas Law Application
This paragraph introduces the application of the ideal gas equation, which states that pressure times volume equals the number of molecules times a constant times the temperature. The speaker explains the relationship between pressure and volume, and how pressure is proportional to the number of particles and temperature. An example problem is presented involving a two-liter balloon filled with hydrogen gas at 30 degrees Celsius and a pressure of two atmospheres, aiming to find the number of moles of hydrogen. The importance of using the correct proportionality constant for the units involved (liters and atmospheres) is emphasized, and the temperature must be converted to Kelvin for accurate calculations.
🔢 Kelvin Temperature Conversion and Gas Law Calculation
The speaker discusses the importance of converting Celsius temperatures to Kelvin when dealing with gas problems in thermodynamics. The paragraph explains the concept of Kelvin as an absolute temperature scale, with 0 Kelvin representing absolute zero. The conversion process from Celsius to Kelvin is described, and the speaker calculates the Kelvin equivalent of 30 degrees Celsius as 303 Kelvin. The paragraph continues with the selection of the appropriate gas constant (R = 0.082 L·atm/(mol·K)) for the units of liters and atmospheres. Using dimensional analysis, the speaker solves for the number of moles (n) of hydrogen in the balloon, resulting in approximately 0.16 moles. The mass of hydrogen in grams is also calculated, which is 0.32 grams, by multiplying the number of moles by the molar mass of hydrogen.
🔄 Further Exploration of Gas Law Problems
In this brief paragraph, the speaker indicates a plan to continue exploring more problems related to the ideal gas law, emphasizing the importance of understanding and correctly using different units in such calculations. The speaker hints at the complexity that can arise from using various units like meters cubed or kilopascals instead of liters or atmospheres, and promises to demonstrate how to select and apply the correct constants for these different units in future examples.
Mindmap
Keywords
💡Ideal Gas Equation
💡Pressure
💡Volume
💡Moles
💡Temperature
💡Kelvin
💡Hydrogen Gas
💡Diatomic Molecule
💡Proportionality Constant
💡Dimensional Analysis
💡Molar Mass
Highlights
The ideal gas equation is introduced, illustrating the relationship between pressure, volume, number of molecules, and temperature.
The concept that pressure is inversely proportional to volume and directly proportional to the number of particles and temperature is explained.
A practical problem involving a two-liter balloon filled with hydrogen gas at 30 degrees Celsius and 2 atmospheres of pressure is presented.
The importance of applying the ideal gas equation to real-world problems is emphasized for a deeper understanding.
The necessity of using the correct proportionality constant for the units of liters and atmospheres is discussed.
The conversion of temperature from Celsius to Kelvin is highlighted as a crucial step in gas law calculations.
A review of the Kelvin temperature scale and its significance in thermodynamics is provided.
The calculation of the number of moles of hydrogen using the ideal gas equation is demonstrated step by step.
The use of dimensional analysis to ensure units cancel out appropriately in the calculation is shown.
The calculation results in 0.16 moles of H2, showcasing the application of the ideal gas law to find the quantity of gas.
An explanation of how to convert moles to the number of molecules using Avogadro's number is given.
The mass of hydrogen gas is calculated based on the number of moles, with a mole of H2 weighing 2 grams.
The practical application of the ideal gas law in determining the mass of a gas in grams is illustrated.
The video promises to cover more problems involving different units to ensure comprehensive understanding.
The importance of selecting the appropriate constant based on the units used in the problem is reiterated.
The video concludes with a reminder of the simplicity of the math involved in gas law problems, emphasizing the importance of unit consistency.
Transcripts
Browse More Related Video
9.2 Gas Laws including the Ideal Gas Law | High School Chemistry
Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion
Ideal gas equation example 3 | Chemistry | Khan Academy
Ideal gas equation example 2 | Chemistry | Khan Academy
Gas Stoichiometry Problems

Ideal Gas Law
5.0 / 5 (0 votes)
Thanks for rating: