9.2 Gas Laws including the Ideal Gas Law | High School Chemistry
TLDRThis chemistry lesson delves into the fundamental gas laws, including Boyle's, Charles's, Avogadro's, the combined gas law, and the ideal gas law. The instructor explains each law's relationship between pressure, volume, temperature, and moles of gas, using the ideal gas law equation as a foundation. The video offers practical examples and calculations, emphasizing the importance of unit consistency and the significance of the Kelvin temperature scale. It also clarifies when to apply the ideal gas law versus the combined gas law in various scenarios.
Takeaways
- 📚 The lesson covers Boyle's Law, Charles's Law, Avogadro's Law, the Combined Gas Law, and the Ideal Gas Law as part of a high school chemistry curriculum.
- 🔬 Boyle's Law states that the pressure of a gas is inversely proportional to its volume when temperature and the number of moles are held constant.
- 🌡️ Charles's Law indicates that the volume of a gas is directly proportional to its temperature when pressure and the number of moles are constant.
- 🧪 Avogadro's Law reveals that the volume of a gas is directly proportional to the number of moles when pressure and temperature are constant.
- 🔄 The Combined Gas Law is a consolidation of Boyle's, Charles's, and Avogadro's Laws, showing the relationship between pressure, volume, temperature, and moles of gas.
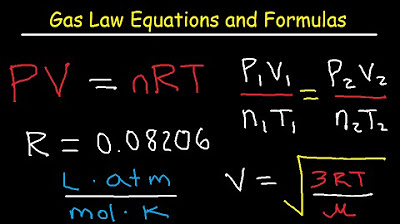
- 🎯 The Ideal Gas Law is represented by the equation PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the universal gas constant, and T is temperature in Kelvin.
- 📉 The Ideal Gas Law can be used to calculate one variable when the other three are known, such as finding the volume of a gas given its pressure, moles, and temperature.
- 📈 The Combined Gas Law is applicable when dealing with changes in two sets of conditions, such as when the pressure and temperature of a gas change.
- 🔢 The universal gas constant (R) has different values depending on the units used, with 1.08206 L·atm/(mol·K) being commonly used in high school chemistry for calculations involving liters and atmospheres.
- 🔄 Understanding the relationship between the variables in the gas laws is crucial for solving problems, such as converting between units and applying the laws to real-world scenarios like a hot air balloon's behavior.
- 📝 The script emphasizes the importance of using the correct units and constants when performing gas law calculations, particularly the Kelvin scale for temperature.
Q & A
What are the four gas laws discussed in the script?
-The four gas laws discussed are Boyle's Law, Charles's Law, Avogadro's Law, and the Ideal Gas Law.
What is the mathematical expression of the Ideal Gas Law?
-The Ideal Gas Law is expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the universal gas constant, and T is temperature in Kelvin.
What does Boyle's Law state in terms of the relationship between pressure and volume?
-Boyle's Law states that the product of pressure (P) and volume (V) is constant when the number of moles (n) and temperature (T) are held constant, or expressed as P1V1 = P2V2.
How does Charles's Law relate volume and temperature for a gas?
-Charles's Law states that the volume (V) of a gas is directly proportional to its temperature (T) when the pressure (P) and the number of moles (n) are held constant, or expressed as V1/T1 = V2/T2.
What is the significance of Avogadro's Law in the context of gas behavior?
-Avogadro's Law states that the volume (V) of a gas is proportional to the number of moles (n) when the pressure (P) and temperature (T) are held constant, or expressed as V1/n1 = V2/n2.
What is the combined gas law and how does it relate to the individual gas laws?
-The combined gas law is an equation that combines Boyle's, Charles's, and Avogadro's Laws into one, expressed as (P1V1/T1) = (P2V2/T2). It is used when the number of moles of gas is constant and allows for the comparison of two different sets of conditions for a gas.
What is the 'better combined gas law' and when is it used?
-The 'better combined gas law' is an extension of the combined gas law that accounts for changes in the number of moles of gas, expressed as (P1V1/n1T1) = (P2V2/n2T2). It is used when parts of two sets of conditions are given, including changes in the amount of gas.
What is the importance of the universal gas constant (R) in the Ideal Gas Law?
-The universal gas constant (R) is crucial in the Ideal Gas Law as it relates the pressure, volume, and temperature of a gas to the number of moles. It has specific values depending on the units used, and it ensures the units on both sides of the equation are balanced.
Why is it necessary to convert temperatures to Kelvin when using the Ideal Gas Law?
-Temperatures must be converted to Kelvin when using the Ideal Gas Law because the Kelvin scale is an absolute temperature scale that starts at absolute zero. This is necessary for accurate calculations and because the gas constant (R) is defined per Kelvin.
How can the Ideal Gas Law be used to calculate the volume of a gas given the number of moles, pressure, and temperature?
-The volume of a gas can be calculated using the Ideal Gas Law rearranged to solve for V: V = nRT/P. Given the number of moles (n), pressure (P), and temperature (T in Kelvin), the volume (V) can be determined by dividing nRT by P.
What is the difference between using the Ideal Gas Law and the combined gas law for calculations?
-The Ideal Gas Law requires one set of conditions with one variable missing to calculate the unknown variable. In contrast, the combined gas law requires parts of two different sets of conditions to calculate an unknown variable, such as when changes in both pressure and temperature occur.
Outlines
📚 Introduction to Gas Laws
This paragraph introduces the topic of gas laws, specifically Boyle's Law, Charles's Law, Avogadro's Law, the Combined Gas Law, and the Ideal Gas Law. It is part of a high school chemistry series released weekly during the 2020-21 school year. The presenter emphasizes that these laws compare two variables at a time and are foundational to understanding the behavior of gases. The Ideal Gas Law is presented as the culmination of these individual laws, relating pressure, volume, the number of moles, and temperature with the universal gas constant. The paragraph also explains that each gas law holds constant two of the four variables involved in the Ideal Gas Law, allowing for the study of the relationship between the remaining two.
🔍 Boyle's and Charles's Laws Explained
This section delves into Boyle's Law and Charles's Law, explaining how they relate volume to pressure and temperature, respectively, while holding the other variables constant. Boyle's Law is expressed in different ways, including the constant product of pressure and volume, and Charles's Law is similarly detailed, with an emphasis on the direct proportionality between volume and temperature. The molecular perspective on gas behavior is introduced to explain these laws, highlighting how increased temperature leads to greater molecular motion and volume expansion.
📉 Avogadro's Law and the Combined Gas Law
Avogadro's Law is discussed, which relates the volume of a gas to the number of moles while keeping pressure and temperature constant. The paragraph uses a humorous anecdote involving Mr. Avogadro and Mr. Boyle to illustrate the concept of proportionality between volume and moles. It then transitions to the Combined Gas Law, which integrates the conditions of all three laws (Boyle's, Charles's, and Avogadro's) to compare different sets of gas conditions, assuming the number of moles is constant. The 'better combined gas law' is introduced to account for changes in the number of moles, expanding the applicability of the law.
🔢 Gas Law Calculations and the Universal Gas Constant
The paragraph focuses on how to perform calculations using the Ideal Gas Law and the Combined Gas Law, emphasizing the importance of using the correct units and the universal gas constant. Two values for the universal gas constant are presented, with one being more applicable to the context of this chapter. An example calculation using the Ideal Gas Law is provided, demonstrating how to find the volume of a gas given the number of moles, pressure, and temperature. The paragraph also discusses the potential for calculations to become more complex by presenting data in less convenient units, necessitating unit conversions.
🌡️ Applying the Combined Gas Law to Variable Conditions
This section illustrates the application of the Combined Gas Law in scenarios where conditions change, such as variations in pressure and temperature. It contrasts the use of the Ideal Gas Law, which requires a single set of conditions with one variable missing, with the Combined Gas Law, which necessitates parts of two different sets of conditions. An example problem is presented where the initial conditions of a gas sample's volume, pressure, and temperature are given, and the task is to find the new volume after changes in pressure and temperature. The importance of using the Kelvin scale for temperature in calculations is stressed, and a step-by-step approach to solving the problem is outlined.
📘 Conclusion on Gas Law Calculations
The final paragraph wraps up the discussion on gas law calculations, highlighting the difference between using the Ideal Gas Law and the Better Combined Gas Law. It reiterates the conditions under which each law is applicable and provides a summary of the calculations performed in the previous paragraphs. The paragraph emphasizes the importance of understanding the relationships between pressure, volume, temperature, and the number of moles in gas law calculations and concludes with a practical example that demonstrates the slight increase in volume due to a minor temperature change, following a significant pressure increase.
Mindmap
Keywords
💡Gas Laws
💡Boyle's Law
💡Charles's Law
💡Avogadro's Law
💡Combined Gas Law
💡Ideal Gas Law
💡Molar Mass
💡Universal Gas Constant (R)
💡Kelvin Scale
💡Conversion Factors
💡Direct Proportionality
💡Inverse Proportionality
Highlights
Introduction to gas laws, including Boyle's Law, Charles's Law, Avogadro's Law, the Combined Gas Law, and the Ideal Gas Law.
Explanation of the Ideal Gas Law formula and its relation to the four variables: pressure, volume, number of moles, and temperature.
Boyle's Law described as the inverse relationship between pressure and volume while holding the number of moles and temperature constant.
Charles's Law explained as the direct proportionality between volume and temperature at constant pressure and number of moles.
Avogadro's Law illustrating the direct relationship between volume and the number of moles of gas, with constant pressure and temperature.
The Combined Gas Law as a consolidation of Boyle's, Charles's, and Avogadro's Laws, applicable when the number of moles is constant.
The 'better combined gas law' introduced to account for changes in the number of moles of gas.
The importance of using the Kelvin scale for temperature in gas law calculations to avoid errors.
Practical examples of gas law applications, such as Mr. Boyle and Mr. Avogadro's hypothetical balloon experiment.
The significance of the universal gas constant 'R' and its role in connecting the variables in the Ideal Gas Law.
Different units for the universal gas constant 'R' and their relevance to specific chemistry contexts.
A step-by-step guide on how to perform calculations using the Ideal Gas Law with given values for pressure, volume, number of moles, and temperature.
The process of converting units for grams to moles, torr to atmospheres, and Celsius to Kelvin for gas law calculations.
The distinction between using the Ideal Gas Law for one set of conditions and the Combined Gas Law for two sets of conditions.
A detailed example calculation using the 'better combined gas law' to find the new volume of a gas sample under changed conditions.
The impact of temperature changes on volume in gas law calculations and the common misconception about doubling the temperature.
The final calculation result using the 'better combined gas law' and the importance of precise temperature conversions in the process.
Transcripts
Browse More Related Video

Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion
Gas Laws - Equations and Formulas

How to Use Each Gas Law | Study Chemistry With Us
Ideal Gas Problems: Crash Course Chemistry #13

Kinetic Molecular Theory and the Ideal Gas Laws

Ideal gas equation example 1 | Chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: