Ideal Gas Law
TLDRIn this AP Physics essentials video, Mr. Andersen explains the ideal gas law, PV=nRT, where P is pressure, V is volume, n is moles, R is the gas constant, and T is temperature in Kelvin. He discusses the concept of absolute zero and how it's approached in the universe and laboratories. The video demonstrates the macroscopic properties of gases and uses a PHET simulation to illustrate the relationships between pressure and volume (Boyle's Law), and volume and temperature. It highlights the importance of keeping variables constant to observe the effects on others, offering a practical approach to understanding gas behavior.
Takeaways
- 📚 The Ideal Gas Law is expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin.
- ❄️ Absolute zero is defined as 0 Kelvin, which is approximately -273 degrees Celsius, and represents the point where molecular motion stops.
- 🌌 The Boomerang Nebula is one of the coldest places in the universe at around 1 or 2 Kelvin.
- 🔬 In laboratories, temperatures close to absolute zero can be achieved by using lasers to halt molecular motion, leading to a new state of matter.
- 🧪 Macroscopic characteristics of gases that can be measured include pressure (P), volume (V), the number of moles (n), and temperature (T) in Kelvin.
- ⚖️ The relationship between pressure and volume can be visualized like a balance scale; increasing pressure requires decreasing volume to maintain equilibrium.
- 🎈 An experiment can involve measuring the volume of a balloon at different temperatures to observe the effects on size and pressure.
- 📉 Extrapolating from volume-temperature graphs can help determine the temperature at which the volume would be zero, indicating absolute zero.
- 🔍 The PHET simulation tool can be used to measure and visualize the relationships between pressure, volume, number of molecules, and temperature.
- 📊 Boyle's Law, which states that pressure and volume are inversely proportional when temperature and the number of gas molecules are constant, can be demonstrated using simulations.
- 🔥 As temperature increases, the average kinetic energy of gas molecules increases, leading to an expansion in volume if pressure is held constant.
- 📚 Conducting experiments and analyzing data with the PHET simulation helps in understanding the relationships between the variables in the Ideal Gas Law.
Q & A
What is the ideal gas law expressed as?
-The ideal gas law is expressed as PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.
What is the significance of 0 Kelvin in the context of the ideal gas law?
-At 0 Kelvin, theoretically, the molecules of a gas would not be moving. This temperature is also known as absolute zero, which is approximately -273 degrees Celsius.
What is the temperature of the Boomerang Nebula, and how does it relate to absolute zero?
-The Boomerang Nebula has a temperature of around 1 or 2 Kelvin, which is very close to absolute zero, indicating a state where molecular motion is nearly non-existent.
How can a state of matter change when the temperature is lowered to fractions of a degree above absolute zero?
-At temperatures close to absolute zero, molecular motion can be significantly reduced, leading to the formation of a new state of matter.
What are the macroscopic characteristics of a gas that can be measured in the laboratory?
-The macroscopic characteristics of a gas that can be measured include pressure (P), volume (V), the amount of gas in moles (n), and temperature (T) in Kelvin.
Can you explain the concept of a teeter totter used in the script to describe the relationship between pressure and volume?
-The teeter totter analogy is used to describe the inverse relationship between pressure and volume in a gas. If pressure increases, the volume must decrease to balance the scale, and vice versa.
How can one measure the volume of a balloon at different temperatures?
-One can measure the volume of a balloon by wrapping a string around it to measure its circumference and calculate its volume. This can be done at different temperatures, such as in a freezer or a warmer area, to observe changes in volume.
What is the relationship between pressure and volume when the temperature is kept constant, as per Boyle's Law?
-According to Boyle's Law, when the temperature is kept constant, the pressure of a gas is inversely proportional to its volume. If the volume decreases, the pressure increases, and if the volume increases, the pressure decreases.
How does the temperature affect the volume of a gas when pressure and the number of molecules are kept constant?
-When the pressure and the number of molecules are kept constant, an increase in temperature will cause the volume of the gas to increase due to the increased average kinetic energy of the molecules. Conversely, a decrease in temperature will result in a decrease in volume.
What is the significance of the different lines on a pressure-volume graph when temperature is kept constant?
-Each line on a pressure-volume graph represents a different temperature of the gas. The lines show how the pressure and volume are related at various temperatures while keeping other variables constant.
How can one use a PHET simulation to understand the relationships between the variables of the ideal gas law?
-A PHET simulation allows one to control and measure variables such as volume, temperature, pressure, and the number of molecules (n). By keeping some variables constant and changing one at a time, one can observe and analyze the relationships between these variables, such as the inverse relationship between pressure and volume or the direct relationship between volume and temperature.
Outlines
🌡️ Ideal Gas Law and Absolute Zero
In this segment, Mr. Andersen introduces the ideal gas law, expressed as PV = nRT, where P stands for pressure, V for volume, n for the number of moles, R for the gas constant, and T for temperature in Kelvin. He discusses the concept of absolute zero, which is approximately -273 degrees Celsius, and mentions that it has been approached in the universe, such as in the Boomerang Nebula, and in laboratories by using lasers to halt molecular motion. The paragraph also covers macroscopic characteristics of gases that can be measured, such as pressure, volume, amount (in moles), and temperature. Mr. Andersen uses an analogy of a teeter-totter to explain the relationship between pressure and volume, and how changes in these variables affect the balance. He also describes an experiment involving a balloon to demonstrate the relationship between volume and temperature, and how extrapolation from a volume-temperature graph can lead to the concept of absolute zero.
📊 Exploring Gas Laws with PHET Simulation
This paragraph focuses on using the PHET simulation to explore the relationships between the variables in the ideal gas law. Mr. Andersen explains the process of keeping certain variables constant while changing one at a time to observe the effects. He specifically discusses Boyle's Law, which describes the inverse relationship between pressure and volume at constant temperature. Using the simulation, he demonstrates how decreasing the volume of a container increases the pressure and vice versa. He also examines the relationship between volume and temperature, showing that as temperature increases, the volume does as well, due to the increased average kinetic energy of the molecules. The paragraph concludes with a discussion on how to graph these relationships and the importance of understanding the direct relationship between temperature and volume, as well as the concept of extrapolation to absolute zero.
Mindmap
Keywords
💡Ideal Gas Law
💡Kelvin
💡Boyle's Law
💡Moles
💡Gas Constant
💡Temperature
💡Pressure
💡Volume
💡Absolute Zero
💡Molecular Motion
💡Extrapolation
Highlights
The ideal gas law is expressed as PV = nRT, where P stands for pressure, V for volume, n for the number of moles, R for the gas constant, and T for temperature in Kelvin.
Absolute zero is defined as 0 Kelvin, equivalent to -273 degrees Celsius, and is the theoretical point where molecular motion stops.
The closest natural occurrence to absolute zero is the Boomerang Nebula, with temperatures around 1 or 2 Kelvin.
In laboratories, temperatures close to absolute zero have been achieved by using lasers to halt molecular motion.
Macroscopic characteristics of a gas that can be measured include pressure, volume, the amount (in moles), and temperature.
The relationship between pressure and volume can be visualized as a balance, where increasing pressure requires a decrease in volume to maintain equilibrium.
Boyle's Law is mentioned, which studies the relationship between pressure and volume at constant temperature.
A PHET simulation is used to demonstrate the relationship between pressure, volume, and temperature in an ideal gas.
When volume is decreased at constant temperature, pressure increases, as demonstrated in the PHET simulation.
Conversely, increasing the volume at constant temperature results in a decrease in pressure.
Different temperature states of a gas result in different pressure-volume curves when graphed.
The relationship between volume and temperature is direct; as temperature increases, so does the volume, assuming pressure and moles are constant.
An increase in temperature leads to an increase in the average kinetic energy of gas molecules, causing them to move faster and occupy more volume.
Decreasing the temperature results in a decrease in volume, as the kinetic energy of the molecules decreases.
Extrapolating a volume versus temperature graph can help determine absolute zero.
The PHET simulation allows for the manipulation of gas properties to understand the relationships between pressure, volume, and temperature.
Experiments using the PHET simulation should involve keeping some variables constant while changing one at a time to analyze the relationships effectively.
Understanding the pressure-volume relationship is crucial for analyzing the behavior of gases under different conditions.
Transcripts
Browse More Related Video
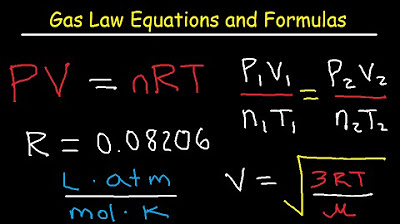
Gas Laws - Equations and Formulas
Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion
Temperature: Crash Course Physics #20

Ideal gas equation example 1 | Chemistry | Khan Academy
Ideal gas equation example 2 | Chemistry | Khan Academy

Kinetic Molecular Theory and the Ideal Gas Laws
5.0 / 5 (0 votes)
Thanks for rating: