Acids and Bases - Basic Introduction - Chemistry
TLDRThis lesson delves into the fundamentals of acids and bases, explaining how to identify them by their chemical formulas and charge states. It covers the Arrhenius, Bronsted-Lowry, and Lewis definitions, highlighting the differences between strong and weak acids and bases, and how they ionize in solution. The importance of pH and its calculation, as well as the autoionization of water and the concept of amphoteric substances, are also discussed. The lesson provides a comprehensive understanding of acid-base reactions, their properties, and practical applications.
Takeaways
- 📌 Acids are compounds that typically have a hydrogen ion (H+) in their formula, such as HCl (hydrochloric acid).
- 📌 Bases often contain a hydroxide ion (OH-), like NaOH (sodium hydroxide), and are usually associated with metals.
- 📌 The Arrhenius definition of acids states that acids release H+ ions, while bases release OH- ions in solution.
- 📌 According to the Bronsted-Lowry definition, acids are proton donors and bases are proton acceptors.
- 📌 The conjugate acid of a substance is formed by adding an H+ to it, increasing its charge by 1.
- 📌 The conjugate base of a substance is formed by removing an H+ from it, decreasing its charge by 1.
- 📌 The pH scale ranges from 0 to 14, with 7 being neutral, below 7 being acidic, and above 7 being basic.
- 📌 Strong acids completely ionize in solution, while weak acids only partially ionize, existing in equilibrium with their ionized forms.
- 📌 Common strong acids include HCl, HBr, HI, HNO3, H2SO4, and HClO4, while weak acids like HF are less common.
- 📌 The strength of an acid is directly related to its Ka value, with higher Ka values indicating stronger acids and lower Ka values indicating weaker acids.
- 📌 The PKa value is the negative logarithm of the Ka value, and it inversely relates to acid strength; lower PKa values correspond to stronger acids.
Q & A
What is the primary characteristic of acids according to the Arrhenius definition?
-According to the Arrhenius definition, acids are substances that release H+ ions (hydrogen ions) into a solution.
How do bases differ from acids in terms of ion release according to the Arrhenius definition?
-In contrast to acids, bases release OH- ions (hydroxide ions) into a solution according to the Arrhenius definition.
What is the role of hydrogen in identifying acids and bases?
-Hydrogen plays a crucial role in identifying acids and bases. Acids typically have a hydrogen ion (H+) in front of them, while bases often have a hydroxide ion (OH-) or a hydrogen ion next to a metal.
How can you distinguish between strong and weak acids based on their ionization?
-Strong acids ionize completely in solution, meaning they dissociate almost entirely into ions. Weak acids, on the other hand, only partially ionize and do not dissociate completely.
What is the significance of the pH scale in understanding the acidity or basicity of a solution?
-The pH scale, ranging from 0 to 14, is used to measure the acidity or basicity of a solution. A pH of 7 indicates a neutral solution, pH less than 7 indicates an acidic solution, and pH greater than 7 indicates a basic solution.
What is the relationship between the concentration of H3O+ ions and the pH of a solution?
-The pH of a solution is the negative logarithm (log) of the concentration of H3O+ ions. As the concentration of H3O+ increases, the pH value decreases, indicating a more acidic solution.
How do you identify the conjugate acid and base in a reaction?
-In an acid-base reaction, the acid donates a proton (H+) and becomes the conjugate base, while the base accepts a proton and becomes the conjugate acid. The conjugate acid is formed by adding an H+ to the base, and the conjugate base is formed by removing an H+ from the acid.
What is the role of temperature in the autoionization constant of water (Kw)?
-The autoionization constant of water (Kw) is dependent on temperature. As the temperature increases, Kw increases, meaning more water molecules ionize into H3O+ and OH- ions. At 25 degrees Celsius, Kw is 1 x 10^-14.
How can you determine if a substance is an amphoteric based on its behavior in reactions?
-An amphoteric substance is one that can behave as both an acid and a base. It can either donate a proton (act as an acid) or accept a proton (act as a base) depending on the reaction conditions and the other reactants involved.
What is the relationship between the Ka and Kb constants in the context of water's self-ionization?
-For water's self-ionization, the equilibrium expression is not Ka or Kb but Kw, which is the autoionization constant for water. At 25 degrees Celsius, Kw is equal to the product of the concentrations of H3O+ and OH- ions, which is 1 x 10^-14. This means that Kw is equal to Ka times Kb at this temperature.
How can you calculate the Pka value of an acid given its Ka value?
-The Pka value of an acid is the negative logarithm (log) of its Ka value. So, to calculate the Pka, you take the negative log of the Ka value. For example, if Ka is 1.8 x 10^-5, then the Pka is the negative log of this number, which is approximately 4.74.
Outlines
📘 Introduction to Acids and Bases
This paragraph introduces the fundamentals of acids and bases, emphasizing the importance of identifying them based on their chemical formulas. Acids are characterized by the presence of hydrogen, exemplified by HCl (hydrochloric acid) and HF (hydrofluoric acid). Bases, on the other hand, contain a hydroxide ion, as seen in NaOH (sodium hydroxide) and KOH (potassium hydroxide). The distinction between acids and bases is further clarified by their charges, with acids typically being positively charged and bases being negatively charged. The paragraph also introduces the Arrhenius and Bronsted-Lowry definitions of acids and bases, as well as the concept of conjugate acids and bases, using examples like HCl and NH3 to illustrate these concepts.
📙 Understanding pH and Acid-Base Reactions
This section delves into the pH scale and its significance in determining the acidity or basicity of a solution. The pH scale ranges from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating basicity. The paragraph explains how to calculate pH through the concentration of hydronium ions and also introduces the concept of pOH, which is related to the concentration of hydroxide ions. The relationship between pH and pOH is highlighted by the fact that their sum equals 14 at 25°C. The discussion then moves to strong and weak acids, differentiating them by their degree of ionization in water and their ability to conduct electricity. Common strong acids are listed, and a pattern is identified regarding the acidity of oxyacids based on the number of oxygen atoms.
📙 Writing Chemical Reactions with Acids and Bases
This paragraph focuses on how to write chemical reactions involving strong acids and weak acids. Strong acids, which ionize completely in water, are represented by single arrows in their reactions, while weak acids, which only partially ionize, are represented by double arrows indicating reversible reactions. The paragraph also discusses the identification and writing of strong and weak bases, with examples of soluble ionic compounds as strong bases and insoluble compounds as weak bases. The mechanisms of reactions involving oxides and hydrides are explained, showing how they behave as strong bases in water.
📘 Properties of Acids and Bases
This section reviews the physical properties of acids and bases, such as their sour taste and slippery feel, respectively. It also covers the use of indicators like litmus paper to identify acids and bases based on color changes. The electrical conductivity of acidic and basic solutions is discussed, with strong acids and bases being strong electrolytes and weak acids and bases being weak electrolytes. The reaction of active metals with acids to produce hydrogen gas is also mentioned, highlighting the reactivity of certain metals with acids. The paragraph concludes with a review of the various definitions of acids and bases, including Arrhenius, Bronsted-Lowry, and Lewis definitions.
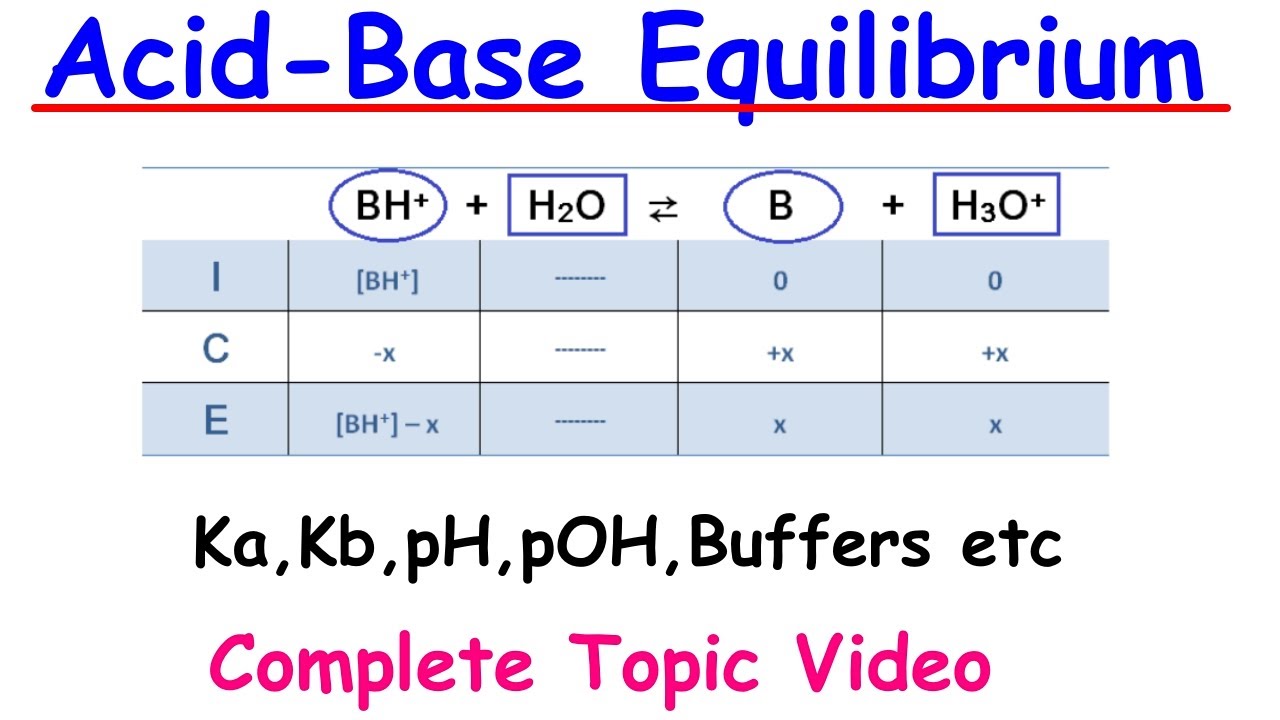
📙 Dissociation Constants and Amphoteric Substances
This paragraph introduces the concept of dissociation constants (Ka and Kb) for acids and bases, explaining how these constants relate to the strength of an acid or base. The calculation of Ka and pKa values for weak acids is discussed, as well as the calculation of Kb and pKb values for weak bases. The relationship between Ka, Kb, and the autoionization constant of water (Kw) is highlighted, with the inverse relationship between Ka and pKa, and Kb and pKb, being emphasized. The paragraph also explores the concept of amphoteric substances, which can act as both acids and bases depending on the reaction conditions, with water and H2PO4^- being used as examples.
📙 Practice Problems and pH Calculations
This section provides a series of practice problems related to calculating pH, pOH, and the concentrations of hydronium and hydroxide ions in a solution. The problems involve applying the formulas and concepts learned in the previous paragraphs, such as the relationship between pH and pOH (summing up to 14), the calculation of hydroxide ion concentration from hydronium ion concentration (using Kw), and vice versa. The problems also touch on the calculation of Ka and Kb values from given concentrations and the determination of the strength of acids and bases based on these values.
📘 Strength of Acids and Bases
This paragraph discusses the relationship between the strength of acids and bases and their respective Ka and Kb values. It explains that a stronger acid will have a higher Ka value and a lower pKa value, while a stronger base will have a higher Kb value. The paragraph uses the Ka values of HF and acetic acid to illustrate which is the stronger acid and discusses the corresponding conjugate bases, explaining that the stronger the acid, the weaker its conjugate base. The concept is further reinforced by calculating the Kb values for the conjugate bases of HF and acetic acid and comparing their strengths.
📘 Lewis Acids and Bases
This section focuses on the Lewis definition of acids and bases, which involves the transfer of electron pairs. Lewis acids are electron pair acceptors, while Lewis bases are electron pair donors. The paragraph provides examples to illustrate this definition, such as the reaction between aluminum chloride and ammonia, where ammonia acts as a Lewis base by donating an electron pair to aluminum, making it the Lewis acid. The paragraph also discusses the behavior of metal ions with high positive charges in water, explaining that they can act as Lewis acids and result in acidic solutions.
Mindmap
Keywords
💡Acids
💡Bases
💡Arrhenius Definition
💡Bronsted-Lowry Definition
💡Conjugate Acid-Base Pairs
💡pH Scale
💡Strong and Weak Acids
💡Electrolytes
💡Amphoteric Substances
💡Ka and Kb
💡Autoionization of Water
Highlights
The lesson focuses on the basics of acids and bases, including how to identify them from their chemical formulas.
Acids typically have a hydrogen element in their formula, such as HCl (hydrochloric acid) and HF (hydrofluoric acid).
Bases often contain a hydroxide ion and are identified by the presence of metal elements, like NaOH (sodium hydroxide) and KOH (potassium hydroxide).
The Arrhenius definition of acids involves substances that release H+ ions into a solution, while bases release hydroxide ions.
Bronsted-Lowry definition describes acids as proton donors and bases as proton acceptors.
In an acid-base reaction, the acid turns into the conjugate base and the base turns into the conjugate acid.
The pH scale ranges from 0 to 14, with 7 being neutral, below 7 being acidic, and above 7 being basic.
The concentration of hydronium ions (H3O+) is used to calculate the pH of a solution, and the concentration of hydroxide ions (OH-) is used to calculate the pOH.
Strong acids ionize completely in solution, while weak acids only partially ionize.
Common strong acids include HCl, HBr, HI, HNO3, H2SO4, and HClO4, with HF being a weak acid.
Oxyacids with more oxygen atoms tend to be stronger acids, such as sulfuric acid being stronger than sulfurous acid.
The chemical reactions of strong acids with water are represented with a single arrow, while weak acids use a double arrow due to reversible reactions.
Strong bases are soluble ionic compounds that ionize virtually completely, whereas weak bases are associated with insoluble compounds and ionize less.
Ammonia and the conjugate bases of weak acids are examples of weak bases.
The mechanism of reactions involving oxides and hydrides in water involves the transfer of hydrogen atoms and the formation of hydroxide ions.
Acids taste sour and turn blue litmus paper red, while bases taste bitter and turn red litmus paper blue.
Both acids and bases can conduct electricity in solution due to the ionization process.
The autoionization constant of water (Kw) is dependent on temperature, with higher temperatures increasing the ionization of water molecules.
The relationship between Ka, Kb, and Kw is such that Ka × Kb = Kw, which can be used to calculate the Kb value from a known Ka and vice versa.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: