16.1 Introduction to Acids and Bases | General Chemistry
TLDRThis lesson delves into the fundamentals of acids and bases, exploring three key definitions: Arrhenius, Bronsted-Lowry, and Lewis. It distinguishes between strong and weak acids and bases based on their degree of dissociation in water. The concept of conjugate acids and bases is introduced, emphasizing their roles in equilibrium and their significance in determining the strength of an acid or base. The video also highlights the importance of recognizing strong acids and bases for effective pH calculations and provides insights into identifying weak acids and bases, with a focus on ammonia and its derivatives as common weak bases.
Takeaways
- 📚 Acids and bases are fundamental concepts in chemistry with three primary definitions: Arrhenius, Bronsted-Lowry, and Lewis.
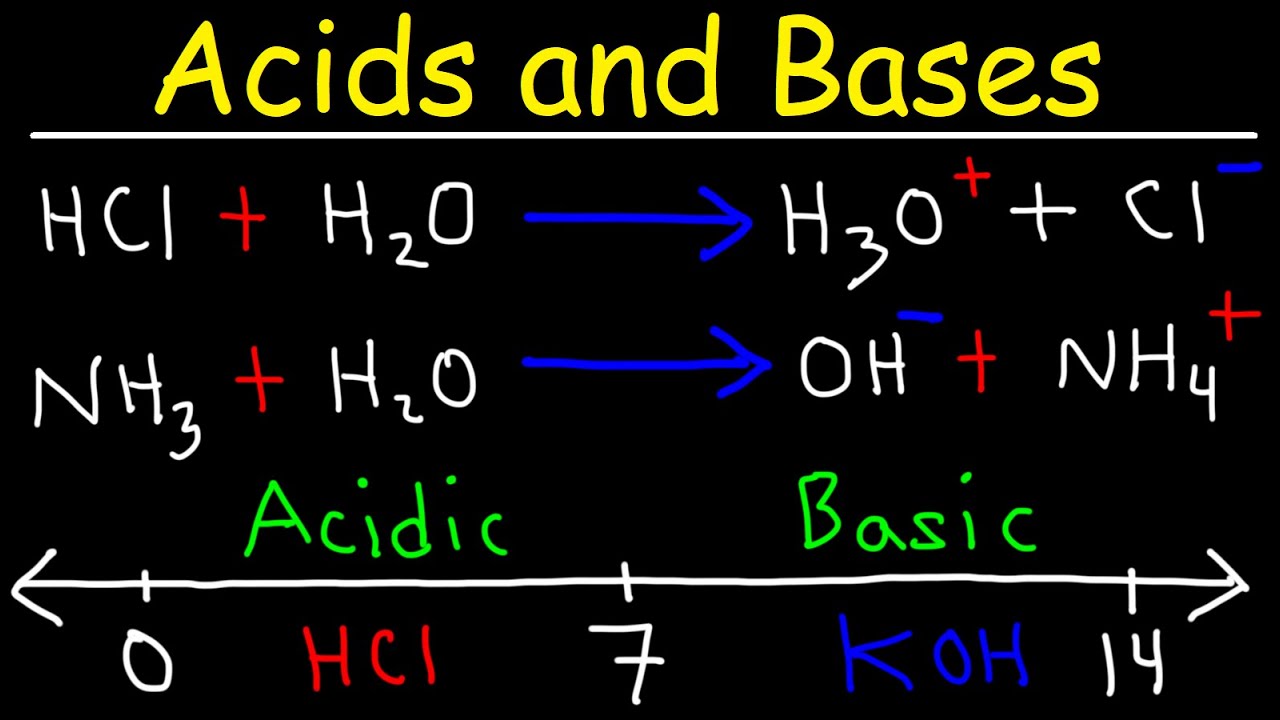
- 💧 The Arrhenius definition is limited to aqueous solutions, where acids increase the concentration of H3O+ and bases increase OH-.
- 🔄 Bronsted-Lowry expanded the definition beyond water, describing acids as proton (H+) donors and bases as proton acceptors.
- 💡 Lewis's definition focuses on electron pairs, with acids as electron pair acceptors and bases as electron pair donors.
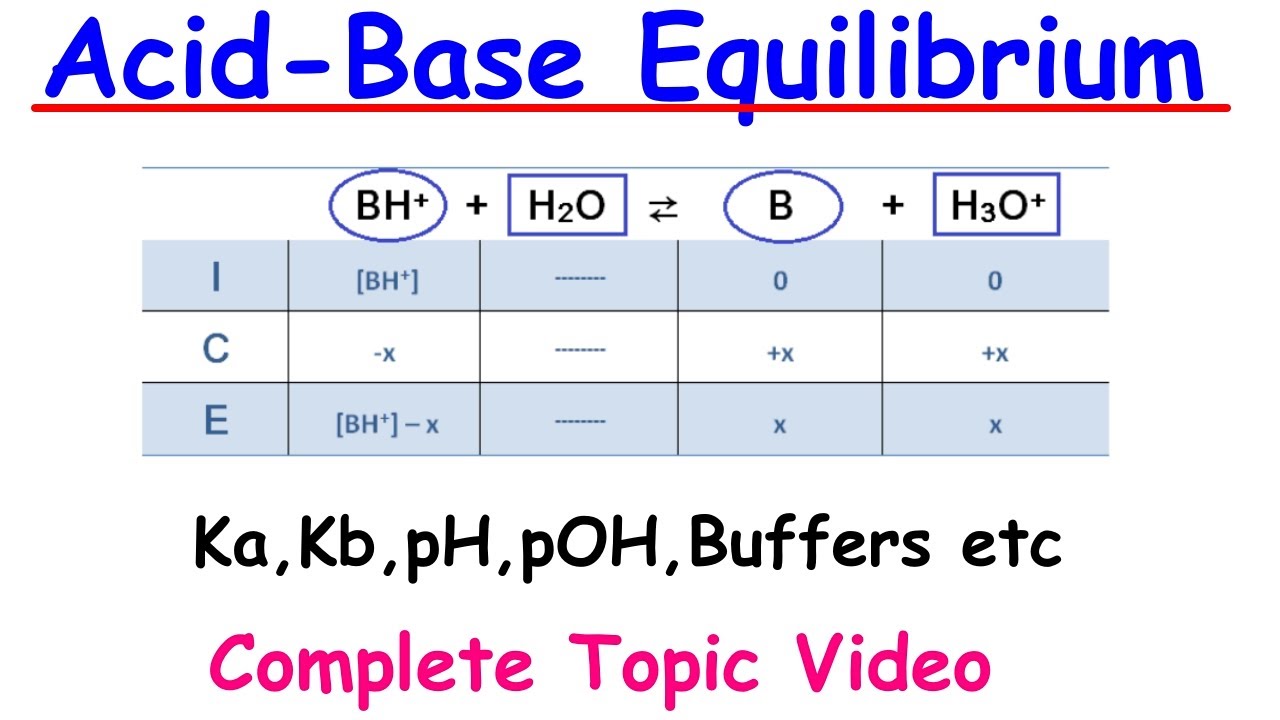
- 🔄 Conjugate acids and bases are pairs formed when an acid donates or a base accepts a proton, with their properties affecting the strength of the original acid or base.
- 🌡️ Strong acids and bases dissociate completely in water, while weak acids and bases only partially dissociate, affecting their pH and behavior in solutions.
- 🥊 Recognizable strong acids include HCl, HI, HBr, and HClO4, among others, and strong bases include Group 1 metal hydroxides and most Group 2 metal hydroxides.
- 🧪 Common weak acids include HF and acetic acid, while weak bases are often ammonia and its organic derivatives.
- 🔍 Identifying the strength of an acid or base is crucial for understanding pH calculations and the behavior of substances in chemical reactions.
- 📈 The dissociation of weak acids is concentration dependent, with higher concentrations resulting in less dissociation and vice versa.
- 🎓 Understanding the distinctions between different types of acids and bases is essential for general chemistry and more specialized studies.
Q & A
What are the three definitions of acids and bases discussed in the lesson?
-The three definitions of acids and bases discussed in the lesson are the Arrhenius definition, the Bronsted-Lowry definition, and the Lewis definition. The Arrhenius definition limits acids and bases to aqueous solutions, where acids increase the concentration of H3O+ and bases increase the concentration of OH-. The Bronsted-Lowry definition broadens the scope to any proton donor as an acid and any proton acceptor as a base, not necessarily in aqueous solutions. The Lewis definition further expands the concept by defining acids as electron pair acceptors and bases as electron pair donors, applicable to reactions not involving hydrogen as well.
How does the Arrhenius definition of acids and bases differ from the Bronsted-Lowry definition?
-The Arrhenius definition is limited to aqueous solutions, where acids are substances that increase the concentration of H3O+ ions and bases are substances that increase the concentration of OH- ions in water. In contrast, the Bronsted-Lowry definition does not require the presence of water and considers any substance that donates a proton (H+) as an acid and any substance that accepts a proton as a base.
What is the significance of the term 'conjugate acid' in the context of Bronsted-Lowry theory?
-In the context of Bronsted-Lowry theory, a 'conjugate acid' refers to the species formed when a base accepts a proton (H+). It is the product of the base's reaction with a proton, and it is the complementary partner to the original base in a proton transfer reaction.
How does the strength of an acid or base affect its dissociation in water?
-The strength of an acid or base greatly influences its degree of dissociation in water. Strong acids and bases dissociate completely in water, meaning they fully ionize into their respective ions. Weak acids and bases, on the other hand, only partially dissociate, with a significant portion remaining as undissociated molecules in solution.
What are some examples of strong acids mentioned in the lesson?
-The lesson mentions several strong acids, including hydrochloric acid (HCl), nitric acid (HNO3), sulfuric acid (H2SO4), perchloric acid (HClO4), hydrobromic acid (HBr), and hydriodic acid (HI). There is also a mention of chloric acid (HClO3), which is sometimes considered a strong acid.
How does the Bronsted-Lowry definition apply to the reaction between HCl and NH3?
-According to the Bronsted-Lowry definition, in the reaction between HCl and NH3, HCl acts as an acid by donating a proton (H+) to NH3, which acts as a base by accepting the proton. This results in the formation of NH4+ (the conjugate acid of NH3) and Cl- (the conjugate base of HCl).
What is the difference between a Lewis acid and a Lewis base?
-A Lewis acid is an electron pair acceptor, meaning it accepts a pair of electrons from another species to form a new bond. A Lewis base, on the other hand, is an electron pair donor, providing a pair of electrons to form a new bond with a Lewis acid. This definition extends beyond protons and includes reactions with other atoms as well.
How can you identify the conjugate base of a given acid?
-To identify the conjugate base of a given acid, you consider the acid donating a proton (H+). The remaining species after the acid donates the proton is its conjugate base. For example, the conjugate base of HCl is Cl-, as it is the species left after HCl donates its proton.
What is the leveling effect in the context of strong acids in water?
-The leveling effect refers to the phenomenon where strong acids, when dissolved in water, all appear to have the same strength because they dissociate completely, resulting in the formation of H3O+ ions. Despite the intrinsic differences in their strengths, the effect of these acids in water is the same due to this complete dissociation.
What are some examples of weak bases mentioned in the lesson?
-The lesson mentions ammonia (NH3) as a common weak base. It also alludes to organic amines, such as methylamine (CH3NH2), which are derivatives of ammonia and are also considered weak bases. These nitrogen-containing compounds are classic examples of weak bases.
How does the structure of a compound influence whether it is an acid or a base?
-The structure of a compound significantly influences its acidic or basic properties. For example, compounds with a COOH group at the end of the molecule, such as acetic acid, are typically acids. On the other hand, compounds like ammonia (NH3) and its derivatives, which contain a nitrogen atom with a lone pair of electrons, are typically bases. The presence of these functional groups is key to identifying the acidic or basic nature of a compound.
Outlines
📚 Introduction to Acids and Bases
The paragraph introduces the topic of acids and bases, highlighting the three main definitions (Arrhenius, Bronsted-Lowry, and Lewis) and their historical progression. It explains that Arrhenius's definition is limited to aqueous solutions and focuses on the increase of H3O+ concentration, while Bronsted-Lowry's definition broadens the scope to any solvent and considers acids as proton donors and bases as proton acceptors. The Lewis definition further expands the concept by focusing on electron transfer, identifying acids as electron acceptors and bases as electron donors. The paragraph sets the stage for a detailed exploration of these concepts in the lesson.
🧪 Arrhenius and Bronsted-Lowry Acid-Base Reactions
This paragraph delves deeper into the specific reactions of acids and bases according to the Arrhenius and Bronsted-Lowry definitions. It uses the example of HCl in water to illustrate how Arrhenius's definition views acids as substances that increase the concentration of H3O+ ions. The paragraph then contrasts this with Bronsted-Lowry's definition, which allows for reactions in various solvents and gas phases, identifying the acid as the proton donor and the base as the proton acceptor. The explanation includes the reaction of HCl with ammonia in the gas phase to demonstrate the broader application of Bronsted-Lowry's definition.
🔬 Lewis Acid-Base Theory and Examples
The paragraph discusses the Lewis acid-base theory, which broadens the concept of acids and bases beyond proton transfer to electron transfer. Acids are redefined as electron acceptors, and bases as electron donors. The explanation includes a detailed look at how Lewis structures help identify the Lewis acid and base in a reaction, using the reaction between HCl and NH3 as an example. The paragraph also introduces a unique class of Lewis acids, such as boron compounds and metal ions, which do not fit into the previous definitions but can still act as electron acceptors.
🔄 Conjugate Acid-Base Pairs and Amphiprotic Species
This section focuses on the concept of conjugate acids and bases, explaining how they are formed when an acid donates or a base accepts a proton. The paragraph uses HCl and NH3 to illustrate the formation of conjugate pairs and introduces the term 'amphiprotic' for species that can act as both acids and bases. It provides examples of how to identify the conjugate acid or base for different species, including the tricky cases of hydroxide and bicarbonate ions. The explanation emphasizes the importance of understanding these concepts for predicting the behavior of substances in equilibrium.
📈 Strength of Acids and Bases: Strong vs. Weak
The paragraph discusses the difference between strong and weak acids and bases, based on their degree of dissociation in water. Strong acids are identified, including the seven most commonly recognized, and their characteristic of dissociating completely in water is explained. The concept of the leveling effect is introduced, which makes all strong acids appear equally strong in aqueous solutions. The paragraph also covers strong bases, primarily Group 1 and some Group 2 metal hydroxides, and notes the solubility and dissociation challenges with Group 2 hydroxides. The distinction between strong and weak acids and bases is crucial for understanding pH calculations and the behavior of substances in solution.
🌿 Organic Acids and Weak Bases
This section addresses the identification of weak acids and bases, noting the challenge in recognizing weak bases compared to weak acids. The paragraph highlights acetic acid as a common weak acid and explains how to recognize it and other organic acids by their COOH functional group. It also discusses weak bases, with ammonia being a primary example, and notes that other nitrogen-containing compounds resembling ammonia are likely to be weak bases. The importance of recognizing these substances is emphasized in the context of general chemistry and future lessons on pH calculations.
🎓 Conclusion and Study Resources
The final paragraph wraps up the lesson on acids and bases, emphasizing the importance of understanding the differences between strong and weak acids and bases for future study. The instructor encourages viewers to support the channel and offers additional practice questions and resources through his general chemistry master course. The availability of a free trial for the course is mentioned, and viewers are encouraged to utilize these resources for further study and mastery of general chemistry concepts.
Mindmap
Keywords
💡Acids and Bases
💡Arrhenius Definition
💡Bronsted-Lowry Definition
💡Lewis Definition
💡Conjugate Acid-Base Pairs
💡Strong Acids and Bases
💡Weak Acids and Bases
💡Amphoteric
💡Leveling Effect
💡pH Scale
💡Chemical Equilibrium
Highlights
Introduction to acids and bases as the main topic of the lesson.
Three definitions for acids and bases: Arrhenius, Bronsted-Lowry, and Lewis.
Arrhenius definition limits acids and bases to aqueous solutions and focuses on the increase in H3O+ concentration for acids and OH- concentration for bases.
Bronsted-Lowry definition broadens the scope beyond water, considering any proton (H+) donor as an acid and any proton acceptor as a base.
Lewis definition further expands the concept, defining acids as electron pair acceptors and bases as electron pair donors, not limited to reactions involving hydrogen.
Example of HCl as a strong acid increasing H3O+ concentration in water.
NaOH as a strong base, dissociating to produce OH- ions and increasing hydroxide concentration.
Explanation of conjugate acids and bases, and how they relate to the strength of acids and bases.
Demonstration of how to identify conjugate acid-base pairs using Bronsted-Lowry's definition.
Discussion of amphiprotic species that can act as both acids and bases, such as bicarbonate.
Identification of strong acids including HCl, HI, HBr, and the leveling effect in aqueous solutions.
Listing of strong bases primarily as hydroxide salts of Group 1 and some Group 2 metals.
Explanation of the difference between strong and weak acids and bases in terms of dissociation.
Memorization of strong acids and bases for quick identification of weak counterparts.
Acetic acid (CH3COOH) as an example of a weak acid that doesn't start with 'H'.
Ammonia and its structural derivatives as examples of weak bases.
The importance of recognizing strong and weak acids and bases for pH calculations and understanding their behavior in solutions.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: