Acid Base Introduction
TLDRThe video script discusses the autoionization of water and introduces the concept of acids and bases, focusing on the Arrhenius definition where acids increase hydrogen ion concentration and bases increase hydroxide ion concentration in water. It explains the importance of pH, the influence of temperature on water's autoionization, and provides examples of strong acids and bases. The script also touches on broader definitions of acids and bases, such as the Bronsted-Lowry and Lewis definitions, which extend beyond aqueous solutions and involve proton and electron transfers, respectively.
Takeaways
- 🌊 Autoionization of water: Water can autoionize, forming hydronium (H3O+) and hydroxide (OH-) ions in an equilibrium state.
- ⚖️ Equilibrium and concentration: The concentrations of hydronium and hydroxide ions in pure water at 25°C are important, with a 1:1 ratio and a concentration of 10^-7 M.
- 📌 pH definition: pH is defined as the negative logarithm (base 10) of the hydrogen ion concentration, with pure water having a pH of 7 at 25°C.
- 🔄 Le Chatelier's Principle: Adding substances can shift the autoionization equilibrium, affecting hydrogen and hydroxide ion concentrations.
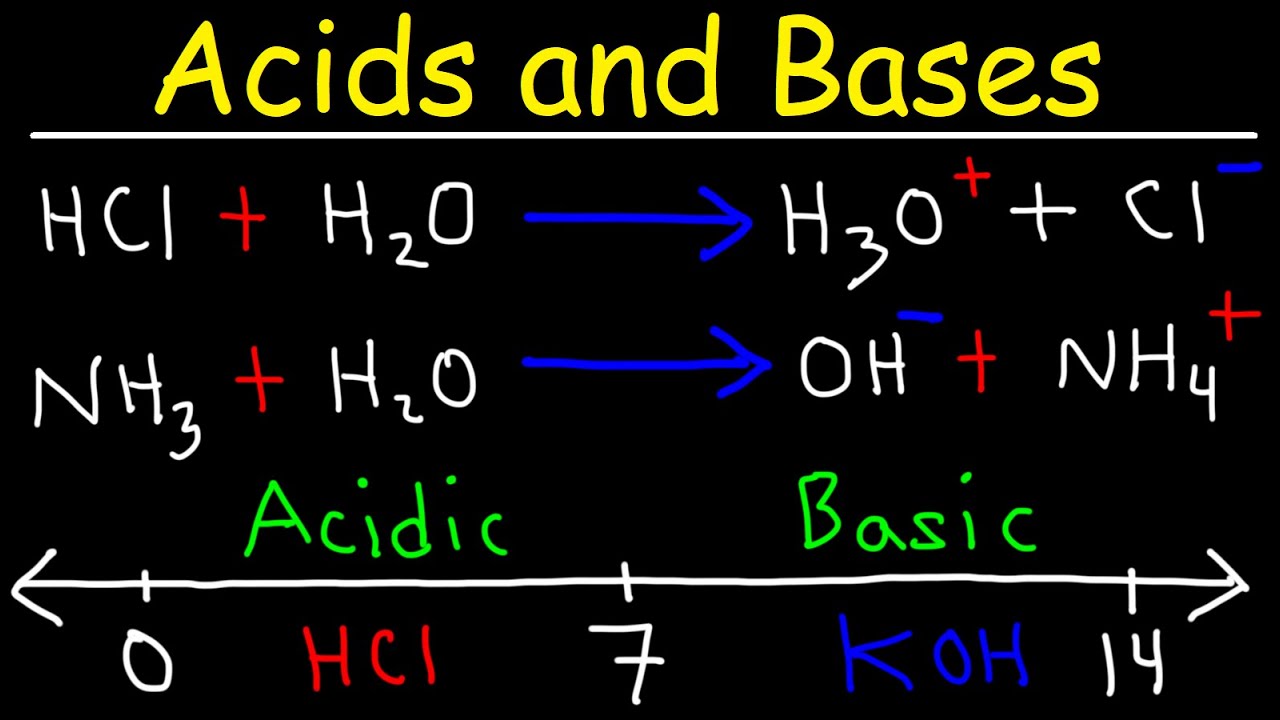
- 🍋 Arrhenius definition: An acid increases hydrogen ion concentration, while a base increases hydroxide ion concentration in an aqueous solution.
- 💧 Strong acids: Hydrochloric acid, hydrogen bromide, hydrogen iodide, nitric acid, sulfuric acid, and perchloric acid are examples of strong acids that completely dissociate in water.
- 🔋 Strong bases: Strong bases, like lithium and sodium hydroxide, dissociate completely in water to form hydroxide ions and their respective cations.
- 🎓 Bronsted-Lowry definition: Acids are proton donors, and bases are proton acceptors, applicable beyond just aqueous solutions.
- 💡 Lewis definition: Lewis acids accept electron pairs, while Lewis bases donate electron pairs, focusing on electron transfer rather than proton transfer.
- 📚 Importance of understanding acid-base definitions: Knowing the different definitions of acids and bases is crucial for chemistry, especially for first-year chemistry students.
- 🔢 Future application: Understanding these concepts will help in future calculations involving pH changes due to the addition of acids or bases.
Q & A
What is autoionization of water?
-Autoionization of water refers to the process where water molecules react with themselves to form a hydronium ion (H3O+) and a hydroxide ion (OH-). This occurs due to the collision of water molecules, where a hydrogen atom from one water molecule transfers to another, creating an ion with an extra hydrogen (hydronium) and one that has lost a hydrogen (hydroxide).
What is the equilibrium constant for the autoionization of water?
-The equilibrium constant for the autoionization of water, often denoted as Kw, is a measure of the extent to which water dissociates into hydronium and hydroxide ions. At 25 degrees Celsius, the value of Kw is 1.0 x 10^-14, indicating that at this temperature, the product of the concentrations of hydronium ions and hydroxide ions in pure water is always this constant value.
What is the significance of the pH scale?
-The pH scale is a logarithmic scale used to specify the hydrogen ion concentration or acidity of a solution. It is defined as the negative logarithm (base 10) of the hydrogen ion concentration. The pH scale ranges from 0 to 14, with 7 being neutral, values below 7 indicating acidic solutions, and values above 7 indicating basic or alkaline solutions.
How does the concentration of hydrogen ions in pure water at 25 degrees Celsius affect the pH?
-At 25 degrees Celsius, the concentration of hydrogen ions in pure water is 1.0 x 10^-7 moles per liter. Using the pH scale, which is the negative logarithm of the hydrogen ion concentration, the pH of pure water at this temperature is calculated to be 7, indicating a neutral pH.
What are strong acids and how do they behave in water?
-Strong acids are substances that completely dissociate or ionize in water, releasing all their hydrogen ions into the solution. This results in a high concentration of hydrogen ions, making the solution highly acidic. Examples of strong acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). They are characterized by a one-way reaction, indicating that the dissociation is complete and does not establish an equilibrium.
What are the Arrhenius definitions of acids and bases?
-According to the Arrhenius definition, an acid is a substance that increases the concentration of hydrogen ions (H+) when dissolved in water, while a base is a substance that increases the concentration of hydroxide ions (OH-) in water. This definition is most commonly used in introductory chemistry classes and is based on the behavior of substances in aqueous solutions.
How do strong bases affect the pH of a solution?
-Strong bases, when dissolved in water, release hydroxide ions (OH-), which leads to a decrease in the concentration of hydrogen ions (H+) and an increase in the pH of the solution. This makes the solution more basic or alkaline. Examples of strong bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
What is the Bronsted-Lowry definition of acids and bases?
-The Bronsted-Lowry definition expands upon the Arrhenius definition by stating that an acid is a proton (H+) donor, while a base is a proton acceptor. This definition extends the concept of acids and bases beyond just aqueous solutions and can be applied to reactions in other solvents or even in the gas phase.
How does the Lewis definition of acids and bases differ from the Bronsted-Lowry definition?
-The Lewis definition of acids and bases focuses on electron pairs rather than protons. According to this definition, an acid is an electron pair acceptor, and a base is an electron pair donor. This broadens the scope of acid-base reactions to include reactions that do not involve protons, such as the reaction between boron trifluoride (BF3) and a fluoride ion (F-), where BF3 acts as a Lewis acid and F- acts as a Lewis base.
Why is the pH of skin important for cosmetics?
-The pH of skin is crucial for maintaining its natural barrier function and the overall health of the skin. Cosmetics that are pH-balanced, meaning they have a similar pH to that of the skin, are less likely to disrupt this natural balance and cause irritation or other negative effects. It's important for cosmetic products to maintain the skin's pH to ensure their compatibility and safety for use.
What are some examples of strong acids and their common names?
-Some examples of strong acids include hydrochloric acid (HCl), which is also known as muriatic acid; sulfuric acid (H2SO4); nitric acid (HNO3); and perchloric acid (HClO4). These acids are known for their ability to completely dissociate in water, resulting in a high concentration of hydrogen ions and thus exhibiting strong acidic properties.
What are some examples of strong bases and their common names?
-Examples of strong bases include sodium hydroxide (NaOH), commonly known as caustic soda or lye; potassium hydroxide (KOH), often referred to as potash; and lithium hydroxide (LiOH). These bases dissociate completely in water to produce hydroxide ions, leading to a high pH and strong basic properties.
Outlines
💧 Autoionization of Water and pH
This paragraph discusses the autoionization process of water, where water molecules can lose a hydrogen atom becoming a hydronium ion (H3O+) and a hydroxide ion (OH-). It explains that this equilibrium reaction is significant in an aqueous solution and introduces the concept of the equilibrium constant. The importance of the concentration of hydrogen ions is highlighted, leading to the definition of pH as the negative logarithm of the hydrogen ion concentration. The video emphasizes the relevance of pH in various applications, such as the pH-balanced cosmetics for skin protection.
🍋 Arrhenius Definition of Acids and Bases
The second paragraph introduces the Arrhenius definition of acids and bases. Acids are substances that increase the hydrogen ion concentration when dissolved in water, while bases increase the hydroxide ion concentration. The paragraph provides examples of strong acids, such as hydrochloric acid (HCl), which completely dissociate in water, and strong bases, like lithium hydroxide (LiOH) and sodium hydroxide (NaOH). It also emphasizes that these substances undergo one-way dissociation reactions, not equilibria, resulting in a significant change in ion concentration.
🔄 Bronsted-Lowry and Lewis Acid-Base Theories
This paragraph delves into the Bronsted-Lowry and Lewis definitions of acids and bases. The Bronsted-Lowry definition broadens the concept by stating that acids are proton donors and bases are proton acceptors. The video uses the example of hydrochloric acid donating a proton to water to form hydronium ions, reaffirming its classification as an acid under both Arrhenius and Bronsted-Lowry definitions. The Lewis definition further expands the concept by focusing on electron transfer, where Lewis acids are electron acceptors and Lewis bases are electron donors. The paragraph illustrates this with the reaction between boron trifluoride (BF3) and a fluoride ion (F-), where BF3 acts as an electron acceptor and F- as an electron donor.
📚 Understanding Acid-Base Reactions
The final paragraph emphasizes the importance of understanding the different acid-base theories, especially for first-year chemistry students. It reiterates that the Arrhenius definition is the most straightforward and relevant for most practical applications, especially those involving pH changes in water. The video concludes by encouraging students to be familiar with these definitions to avoid confusion in future studies, and to look forward to upcoming videos that will explore the mathematical aspects of acid-base reactions and their effects on pH levels.
Mindmap
Keywords
💡Autoionization
💡Hydronium Ion
💡Hydroxide Ion
💡pH
💡Arrhenius Acid/Base
💡Le Chatelier's Principle
💡Strong Acid
💡Strong Base
💡Bronsted-Lowry Acid/Base
💡Lewis Acid/Base
💡Equilibrium Constant
Highlights
Water autoionizes, forming hydronium and hydroxide ions in an equilibrium state.
The autoionization reaction of water can be represented as H2O dissociating into H3O+ and OH- in aqueous solution.
At 25 degrees Celsius, the concentration of hydrogen ions in pure water is 10^-7 molars.
The pH scale is defined as the negative log base 10 of the hydrogen ion concentration.
Pure water at room temperature has a pH of 7, making it neutral.
Le Chatelier's Principle can be applied to understand how adding substances affects the autoionization equilibrium of water.
Acids are substances that increase the hydrogen ion concentration when added to water, following the Arrhenius definition.
Bases are substances that increase the hydroxide ion concentration in water, also according to the Arrhenius definition.
Strong acids, such as hydrochloric acid, completely dissociate in water, forming hydrogen ions and their respective anions.
Examples of strong acids include hydrochloric acid, hydrogen bromide, hydrogen iodide, nitric acid, sulfuric acid, and perchloric acid.
Strong bases, like lithium and sodium hydroxide, dissociate completely in water to form hydroxide ions and their respective cations.
The Bronsted-Lowry definition expands the concept of acids and bases to include proton donors and acceptors, not limited to aqueous solutions.
The Lewis definition of acids and bases focuses on electron acceptors and donors, which is a broader concept than Arrhenius or Bronsted-Lowry definitions.
The autoionization of water and the behavior of acids and bases have significant implications in various fields, including cosmetics and biological systems.
Understanding the different definitions of acids and bases is crucial for a comprehensive study of chemistry, particularly in first-year chemistry classes.
The concept of pH is vital in studying the behavior of solutions and their interactions with various substances.
The equilibrium between hydronium and hydroxide ions in water is a fundamental concept in chemistry, with wide-ranging applications.
Transcripts
Browse More Related Video
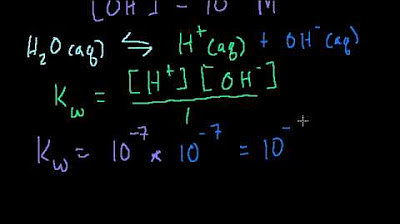
Introduction to pH, pOH, and pKw

Conjugate Acid Base Pairs, Arrhenius, Bronsted Lowry and Lewis Definition - Chemistry

Arrhenius definition of acids and bases | Biology | Khan Academy
Acid Base Equilibrium Full Topic Video
Acids and Bases - Basic Introduction - Chemistry

16.1 Introduction to Acids and Bases | General Chemistry
5.0 / 5 (0 votes)
Thanks for rating: