Acid-Base Equilibrium
TLDRThis chemistry essentials video delves into acid-base equilibrium, explaining the concept of reversible reactions and the significance of the equilibrium constant in understanding acid-base chemistry. It distinguishes between strong and weak acids and bases using their Ka and Kb values, and introduces the concept of titration and buffer solutions. The video also emphasizes the importance of water as an amphoteric solvent in all acid-base reactions and how it influences the pH scale. The equilibrium constant for water, Kw, and its relation to pH and pOH is discussed, along with the characteristics of polyprotic acids and the behavior of strong and weak acids and bases in neutralization reactions.
Takeaways
- 📚 Acid-base equilibrium is a reversible reaction that eventually achieves a state of balance, which can be quantified using the equilibrium constant.
- 🏺 The equilibrium constant (K) is calculated as the concentration of products divided by the concentration of reactants, providing insight into the favorability of the reaction.
- 🔋 Strong acids and bases have a high equilibrium constant (Ka > 1), indicating they favor the formation of products, while weak acids and bases have a low equilibrium constant (Ka < 1), favoring reactants.
- 📈 Titration curves illustrate the change in pH during an acid-base neutralization reaction, with different shapes for strong acid with strong base, strong acid with weak base, and weak acid with strong base.
- 💧 Water plays a crucial role in acid-base chemistry as it is omnipresent and amphoteric, meaning it can act as both an acid and a base.
- 🌀 Autoionization of water is a process where water molecules exchange protons, forming hydronium and hydroxide ions, with a very low equilibrium constant (Kw = 1 × 10^-14).
- 📊 pH and pOH are derived from the equilibrium constant and are related to the concentration of hydronium and hydroxide ions, respectively. They add up to 14 at 25°C.
- 🧪 Acid-base titrations are a method to determine the concentration of an unknown solution by reacting it with a solution of known concentration, using indicators to signal the endpoint.
- 🏛️ The Harvard University Chinese statue example highlights the real-world impact of acid-base chemistry, specifically the damage caused by acid rain to monuments.
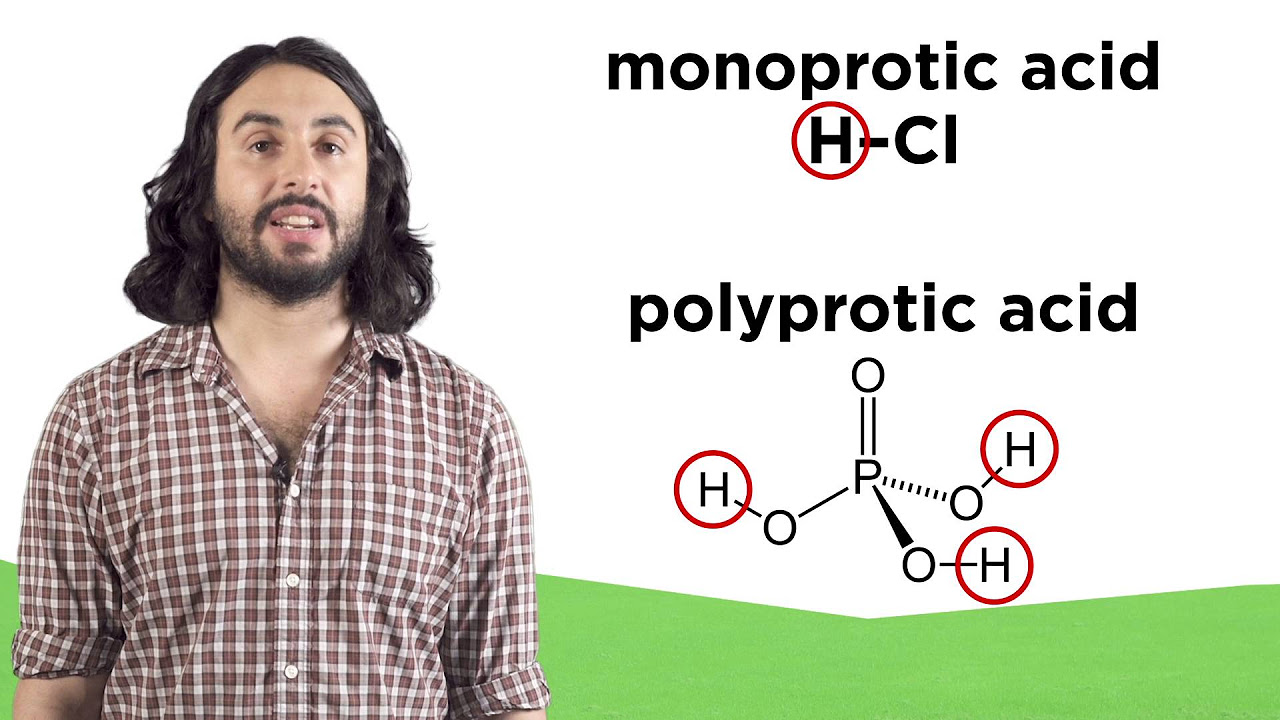
- 🔬 Memorizing the six strong acids and understanding the behavior of polyprotic acids, which can donate more than one proton in a stepwise manner, is important for studying acid-base chemistry.
- 🛠️ Buffer solutions, which stabilize pH against changes, are formed when a weak acid and its conjugate base or a weak base and its conjugate acid are present in significant amounts.
Q & A
What is the main topic of the chemistry essentials video 68?
-The main topic of the video is acid-base equilibrium, including the concepts of acids, bases, and their reactions in the context of equilibrium and titration.
Why is the Chinese statue at Harvard University covered during winter?
-The statue is covered during winter due to the threat of acid rain, which can cause damage to the material of the statue over time.
What is the significance of the equilibrium constant in acid-base chemistry?
-The equilibrium constant helps us understand the balance between reactants and products in acid-base reactions, indicating the extent to which a reaction favors the formation of products or the reformation of reactants.
What defines an acid and a base in the context of the video?
-An acid is a substance that donates a proton, while a base is a substance that accepts a proton. This is related to the proton exchange mechanism in acid-base reactions.
Why is water important in acid-base chemistry?
-Water is crucial in acid-base chemistry because it is the medium in which these reactions occur and it is amphoteric, meaning it can act as both an acid and a base.
What is the autoionization of water and what products does it form?
-The autoionization of water is the process where water serves as both an acid and a base, donating a proton to another water molecule to form hydronium (H3O+) and hydroxide (OH-) ions.
How is the pH of water determined?
-The pH of water is determined by the concentration of hydronium ions, which is equal to the concentration of hydroxide ions due to the autoionization of water. Since their product is 1 x 10^-14, the pH of pure water is 7 at 25°C.
What is the difference between a strong and a weak acid or base based on the equilibrium constant?
-A strong acid or base has an equilibrium constant (Ka for acids, Kb for bases) greater than 1, indicating that the reaction favors the formation of products. A weak acid or base has an equilibrium constant less than 1, meaning the reaction favors the reactants.
What are the six strong acids that should be memorized in AP chemistry?
-The six strong acids to memorize are hydrochloric acid (HCl), sulfuric acid (H2SO4), and the other four can be found in the video or AP chemistry resources.
What is a neutralization reaction and how can it be observed through titration?
-A neutralization reaction is a process where an acid reacts with a base to form water and a salt. It can be observed through titration, where the addition of a base to an acid or vice versa results in a change in pH, which can be graphed to show the equivalence point and buffer region.
How does the pH change during the titration of a strong acid with a strong base?
-During the titration of a strong acid with a strong base, the pH initially remains low but eventually increases and reaches the equivalence point at pH 7, showing a smooth curve without a significant buffer region.
What is the role of Le Chatelier's Principle in the formation of buffer solutions?
-Le Chatelier's Principle explains that when a system at equilibrium is subjected to a change in conditions, the system will adjust to counteract the change and restore equilibrium. In buffer solutions, the addition of more base or acid causes the system to produce more conjugate acid or base to stabilize the pH.
Outlines
📚 Introduction to Acid-Base Equilibrium
This paragraph introduces the concept of acid-base equilibrium, starting with a real-world example of the Chinese statue at Harvard University and its susceptibility to acid rain. The discussion then shifts to the fundamental principles of acid-base chemistry, emphasizing the reversible nature of reactions and the importance of the equilibrium constant in understanding these reactions. The definition of acids and bases is provided, relating to proton exchange, and the role of water as an amphoteric substance in acid-base reactions is highlighted. The paragraph also touches on the concept of conjugate acids and bases, and the autoionization of water, leading to the introduction of the equilibrium constant for water (Kw) and the derivation of pH and pOH values.
🧪 Strong and Weak Acids and Bases
This paragraph delves into the distinction between strong and weak acids and bases, using the equilibrium constant (Ka) as a measure of their strength. Strong acids are described as those that completely donate their protons, resulting in a large Ka value, while weak acids have a small Ka value, indicating a preference for the reactants. The concept of pKa is introduced as a convenient way to deal with small Ka values. The paragraph also explains the behavior of polyprotic acids, which can donate protons in multiple steps. The characteristics of strong bases and their formation from hydroxides of group I and II elements are outlined. The paragraph concludes with an overview of neutralization reactions, including the three possible scenarios involving strong and weak acids and bases, and the resulting titration curves that illustrate the pH changes during these reactions.
Mindmap
Keywords
💡Acid-Base Equilibrium
💡Equilibrium Constant (K)
💡Proton Exchange
💡Conjugate Acid-Base Pairs
💡Amphoteric
💡Neutralization Reaction
💡Titration
💡pH and pOH
💡Strong and Weak Acids and Bases
💡Polyprotic Acids
💡Buffer Solution
Highlights
A Chinese statue at Harvard University is mentioned as an example of how acid rain affects monuments, highlighting the importance of understanding acid-base chemistry.
Acid-base equilibrium is a reversible reaction that can be understood by measuring the equilibrium constant, which provides insight into acid-base chemistry.
The definition of an acid and a base is related to proton exchange, with acids donating protons and bases accepting them.
The equilibrium constant is calculated as the concentration of products divided by the concentration of reactants, which helps in determining the strength of acids and bases.
Water is crucial in acid-base chemistry as it is omnipresent and amphoteric, meaning it can act as both an acid and a base.
A simple reaction example is given with acetic acid in water, where acetic acid acts as an acid and water as a base.
The concept of conjugate acids and bases is introduced, with examples provided for how they are formed in reactions.
Water's ability to autoionize is discussed, leading to the formation of hydronium and hydroxide ions, with their equilibrium constant (Kw) being 1 x 10^-14.
The pH scale is explained as the negative log of the hydronium ion concentration, with a neutral pH of 7 corresponding to equal concentrations of hydronium and hydroxide ions.
The distinction between strong and weak acids is made based on their equilibrium constants, with strong acids having Ka values greater than 1 and weak acids having Ka values less than 1.
pKa is introduced as the negative log of Ka, a convenient way to express the strength of weak acids when their Ka values are very small.
Six strong acids are listed that should be memorized in AP chemistry, including hydrochloric acid and sulfuric acid.
Polyprotic acids are defined as acids that can donate more than one proton, undergoing multiple ionization steps.
The general equations for acids and bases are provided, along with the conditions that define a weak base.
Neutralization reactions are explained as reactions between an acid and a base, with three different scenarios based on the strength of the reactants.
Acid-base titrations are described, including the setup and process of adding a strong base (sodium hydroxide) to a strong acid (hydrochloric acid).
The titration curve for a strong acid and strong base is explained, showing a smooth transition to a basic pH at the equivalence point.
The behavior of a strong base with a weak acid during titration is discussed, highlighting the formation of a buffer solution and a higher equivalence point pH.
The concept of buffer solutions and their role in stabilizing pH during titrations is introduced, with the influence of Le Chatelier's Principle.
The titration curve for a strong acid with a weak base is also explained, noting the lower equivalence point pH.
The video concludes with a recap of the differences between strong and weak acids and bases, the importance of equilibrium constants, and the understanding of titration processes.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: