Acid Base Equilibrium Full Topic Video
TLDRThis video script delves into the fundamentals of acid-base chemistry, exploring the definitions of acids and bases according to Arrhenius and Brønsted-Lowry theories. It distinguishes between strong and weak acids and bases, discusses the concept of conjugate acids and bases, and explains the autoionization of water. The script also covers the calculation of pH and pOH, the ion product constant (Kw), and the dissociation constants (Ka and Kb). Through examples and practice problems, the video aims to solidify the viewer's understanding of acid-base equilibria and their practical applications in various chemical scenarios.
Takeaways
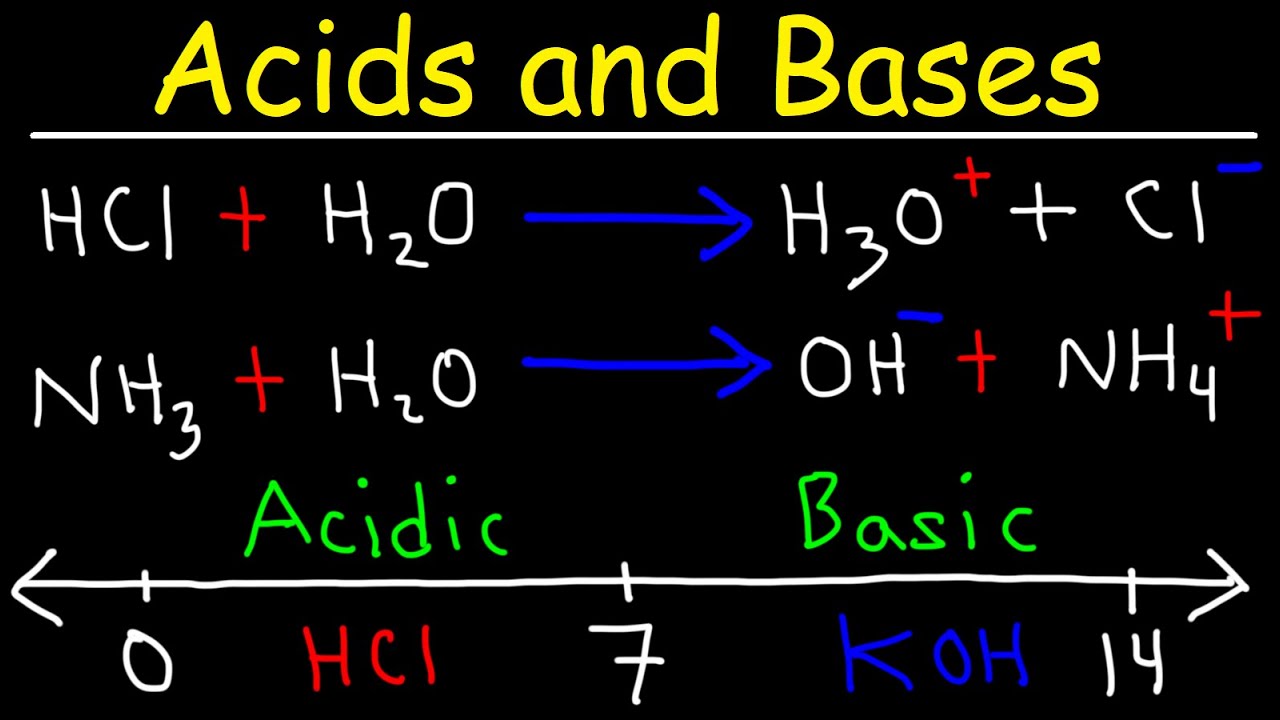
- 📚 The definition of an acid according to Arrhenius is a substance that increases the concentration of hydrogen ions (H+) when dissolved in water.
- 📚 The definition of a base according to Arrhenius is a substance that increases the concentration of hydroxide ions (OH-) when dissolved in water.
- 📚 The Brønsted-Low definition identifies acids as proton (H+) donors and bases as proton acceptors.
- 🧪 Examples of strong acids include hydrochloric acid (HCl) and nitric acid (HNO3), while examples of strong bases include sodium hydroxide (NaOH) and barium hydroxide (Ba(OH)2).
- 📈 The ion product constant of water (Kw) at 25°C is 1 x 10^-14, meaning that the pH and pOH of a neutral solution sum up to 14.
- 🔄 Autoionization of water refers to the ability of water to react with itself to form hydronium (H3O+) and hydroxide (OH-) ions.
- 🧪 The pH scale ranges from 0 to 14, with values below 7 indicating acidity, values above 7 indicating basicity, and a pH of 7 indicating neutrality.
- 📊 The concentration of hydrogen ions in a solution can be calculated from the pH value using the formula: [H+] = 10^(-pH).
- 📈 The percent ionization of a weak acid can be calculated by dividing the concentration of ionized acid by the initial concentration of the acid and multiplying by 100.
- 🧪 The equilibrium constant (Ka or Kb) for an acid or base can be determined from the concentrations of the reactants and products at equilibrium.
- 📚 The video script also discusses methods for accessing educational content, such as through a learning portal or mobile app, and emphasizes the importance of practice in understanding and mastering chemical concepts.
Q & A
What are the two definitions of acids and bases mentioned in the transcript?
-The two definitions of acids and bases mentioned are the Arrhenius (AAS) definition and the Brønsted-Lowry (BL) definition. According to AAS, an acid is a substance that increases the concentration of hydrogen ions (H+) when dissolved in water, while a base is a substance that increases the concentration of hydroxide ions (OH-) in solution. According to BL, an acid is a proton (H+) donor, and a base is a proton acceptor.
How does the Brønsted-Lowry definition differ from the Arrhenius definition?
-The Brønsted-Lowry definition differs from the Arrhenius definition in that it does not require the presence of water or hydroxide ions. Instead, it focuses on the concept of proton transfer, where an acid is a proton donor and a base is a proton acceptor. This broader definition allows for a wider range of reactions and substances to be considered acidic or basic, beyond just those that fit the AAS definition.
What is the significance of the term 'protic' in the context of water?
-The term 'protic' refers to the ability of water to act as both an acid and a base. This is due to its ability to donate a proton (H+) in reactions with bases, and to accept a proton from acids. This dual behavior is a key characteristic of water and is essential for many chemical reactions and equilibria in aqueous solutions.
What is the term used to describe the reverse reaction of a base accepting a proton?
-The term used to describe the reverse reaction where a base accepts a proton is 'conjugate acid'. In this reaction, the base has become a conjugate acid by gaining a proton, and the acid that donated the proton becomes a conjugate base.
How is the ion product constant of water (KW) calculated and what is its value at 25°C?
-The ion product constant of water (KW) is calculated as the product of the concentrations of hydronium ions (H3O+) and hydroxide ions (OH-) in water. At 25°C, the value of KW is 1 x 10^-14. This means that in pure water at this temperature, the concentrations of H3O+ and OH- ions are equal and are 1 x 10^-7 M.
What is the difference between strong and weak acids?
-Strong acids are those that dissociate completely in water, meaning they fully ionize into their constituent ions. Weak acids, on the other hand, only partially dissociate in water, retaining some of their molecular structure and not fully ionizing. This results in a lower concentration of hydrogen ions (H+) for weak acids compared to strong acids at the same initial concentration.
How does the concept of autoionization of water relate to the ion product constant (KW)?
-Autoionization of water refers to the process where water molecules react with each other to form hydronium ions (H3O+) and hydroxide ions (OH-). This process is in equilibrium, and the ion product constant (KW) is the equilibrium constant for this reaction. It represents the product of the concentrations of H3O+ and OH- ions at equilibrium in pure water.
What is the relationship between pH and pOH, and how do they relate to the ion product constant (KW)?
-The pH is the negative logarithm of the hydronium ion concentration (H3O+) and is used to express the acidity of a solution. The pOH is the negative logarithm of the hydroxide ion concentration (OH-) and is used to express the basicity of a solution. The relationship between pH and pOH is given by the equation pH + pOH = 14 at 25°C, which is derived from the ion product constant (KW) of water.
How can the percent ionization of a weak acid be calculated?
-The percent ionization of a weak acid can be calculated by determining the concentration of the hydrogen ions (H+) produced by the acid, dividing it by the initial concentration of the acid, and then multiplying by 100 to get a percentage. This value represents the proportion of the original acid molecules that have donated a proton and ionized.
What is the difference between conjugate acids and bases in the context of a chemical reaction?
-In the context of a chemical reaction, a conjugate acid is the species formed when a base accepts a proton, while a conjugate base is the species formed when an acid donates a proton. These terms are related to the concept that in any acid-base reaction, the acid and base can switch roles; the species that acts as an acid in one reaction can act as a base in another, and vice versa.
How can the equilibrium constant (Ka) for a weak acid be determined experimentally?
-The equilibrium constant (Ka) for a weak acid can be determined experimentally by measuring the pH of a solution of the acid and calculating the concentration of hydrogen ions (H+) at equilibrium. With the known initial concentration of the acid and the equilibrium concentration of H+, the Ka can be calculated using the expression Ka = [H+][A-]/[HA], where [HA] is the initial concentration of the acid, [H+] is the equilibrium concentration of hydrogen ions, and [A-] is the equilibrium concentration of the conjugate base.
Outlines
📚 Introduction to Acid-Base Equilibrium
The paragraph introduces the study of acid-base equilibrium, emphasizing the importance of understanding the definitions of acids and bases. It mentions two definitions, one by Arrhenius and the other by Brønsted-Lowry. The Arrhenius definition describes acids as substances that increase the concentration of hydrogen ions when dissolved in water, while bases increase hydroxide ion concentration. The Brønsted-Lowry definition characterizes acids as proton donors and bases as proton acceptors. The paragraph also explains how to remember these definitions by associating the presence of hydroxide ions with bases and hydrogen ions with acids.
🧪 Examples of Acids and Bases
This section provides examples of both acids and bases, highlighting their chemical properties. Acids such as hydrochloric acid (HCl) and nitric acid have hydrogen attached, while bases like sodium hydroxide (NaOH) and barium hydroxide (Ba(OH)2) contain hydroxide ions. The paragraph explains that these examples align with the definitions provided, where acids donate protons and bases accept them. It also introduces the concept of strong and weak acids and bases, indicating that strong acids and bases dissociate completely in solution, whereas weak ones only partially dissociate.
🌊 Auto-ionization of Water
The paragraph delves into the unique properties of water, discussing its ability to act as both an acid and a base, known as auto-ionization. It explains that water can react with itself to form hydronium (H3O+) and hydroxide (OH-) ions, maintaining a balance in pure water. The ion product constant of water (KW) at 25°C is introduced, and its significance in calculating the concentrations of H3O+ and OH- ions in neutral solutions is highlighted. The paragraph also touches on the pH scale, indicating that a pH of 7 is neutral, below 7 is acidic, and above 7 is basic.
📈 Calculating the Ion Product Constant (KW)
This part of the script focuses on the calculation of the ion product constant (KW) for water at 25°C, which is found to be 1 x 10^-14. The explanation clarifies that in a neutral solution, the concentration of hydronium ions is equal to the concentration of hydroxide ions, and both are represented by the variable 'x'. The calculation involves solving the equation x^2 = 1 x 10^-14, leading to a concentration of 1 x 10^-7 M for both ions in a neutral solution. The relationship between pH and pOH is also discussed, stating that their sum equals 14 at 25°C.
🔬 Methods for Measuring pH
The paragraph discusses various methods for measuring the pH of a solution. It mentions the use of litmus paper, which changes color to indicate whether a solution is acidic or basic. Indicators used in titration are also mentioned, which change color at specific pH levels. The paragraph then introduces the use of a pH meter as a more precise method for measuring pH. The importance of understanding the concepts of acids, bases, and their measurements is emphasized to ensure accurate determination of a solution's pH.
🧬 Dissociation of Weak Acids and Calculation of Ka
This section explains the partial dissociation of weak acids in water, using the example of formic acid. The process involves the loss of a proton (H+) from the acid, forming the conjugate base. The paragraph introduces the concept of the acid dissociation constant (Ka), which is used to calculate the extent of dissociation of weak acids. It also explains how to set up an ICE (Initial, Change, Equilibrium) table to determine the equilibrium concentrations of the species involved in the dissociation reaction, which is crucial for calculating Ka.
📊 Calculation of Percent Ionization
The paragraph discusses the calculation of percent ionization, which indicates the proportion of an acid that has dissociated in solution. It uses the example of formic acid, where the initial concentration and the concentration of dissociated hydrogen ions are known. The calculation involves dividing the concentration of dissociated hydrogen ions by the initial concentration of the acid and multiplying by 100 to get the percentage. The concept is important for understanding the behavior of weak acids in solution and their degree of ionization.
🌐 Accessing Comprehensive Educational Resources
The paragraph provides information on how to access a wide range of educational resources, including full topic videos and practice questions. It mentions the website transcended.institute.net as a platform for accessing these resources. The paragraph also highlights three ways to access the videos: Patreon, Channel memberships, and the learning portal. The resources cover various subjects such as mathematics, physics, chemistry, and biology, and are designed to help learners understand concepts from scratch and prepare for exams.
Mindmap
Keywords
💡Acid
💡Base
💡pH
💡Conjugate Acid-Base Pairs
💡Autoionization of Water
💡Ka and Kb Constants
💡Dissociation
💡Percent Dissociation
💡Strong and Weak Acids and Bases
💡Equilibrium
💡Ion Product Constant (Kw)
Highlights
The introduction of acid-base equilibrium concepts, including definitions of acids and bases according to Arrhenius and Brønsted-Lowry theories.
Explanation of how acids increase the concentration of hydrogen ions and bases increase hydroxide ion concentration in solution.
Discussion on the difference between strong and weak acids and bases, and how their dissociation in water affects ion concentration.
Illustration of the auto-ionization of water, showing water's ability to act as both an acid and a base.
Derivation and explanation of the ion product constant of water (KW) at 25°C, and its significance in determining the pH scale.
Clarification on how to calculate the pH and pOH of a solution, and their relationship to the concentration of hydrogen and hydroxide ions.
Explanation of conjugate acids and bases, and their roles in chemical reactions and equilibrium.
Demonstration of how to write and understand K expressions for acid-base reactions, including the consideration of equilibrium concentrations.
Detailed process of calculating the Ka for a weak acid like formic acid, using the ICE table method and given pH value.
Introduction to various methods for measuring pH, such as litmus paper, indicators, and pH meters, and their practical applications.
Explanation of the relationship between pH and pKa, and how to calculate the latter from the former for a given acid.
Discussion on the percent ionization of acids and how it relates to the initial concentration and the amount of ionized protons.
Overview of the process of acid-base titration, including the importance of understanding conjugate acids and bases in such reactions.
Explanation of how to identify and work with conjugate acid-base pairs in various chemical reactions.
Calculation of pH and pOH for strong acids and bases, and the use of dissociation reactions in determining the concentrations of ions in solution.
Comprehensive guide on finding the K values for both acids and bases, and the application of these values in solving equilibrium problems.
Detailed walkthrough of how to approach and solve questions involving acid-base equilibrium, including the use of the ICE table and the application of logarithms.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: