21. Acid-Base Equilibrium: Is MIT Water Safe to Drink?
TLDRThis lecture delves into the fundamentals of acid-base chemistry, contrasting Lewis and Bronsted-Lowry definitions and exploring the relationship between pKw, pH, and pOH. It discusses the strengths of acids and bases, using examples like NH3 and BF3, and clarifies the distinction between strong and weak acids and bases through Ka and Kb constants. The lecture also covers practical aspects, such as measuring pH with paper and the importance of pH in everyday substances, emphasizing the relevance of acid-base chemistry in various fields.
Takeaways
- 📚 The lecture discusses the definitions of Lewis and Bronsted-Lowry acids and bases, highlighting their compatibility and differences in the context of chemical reactions.
- 🔬 It emphasizes the importance of understanding the equilibrium constant (K) in acid-base reactions, especially in relation to the ionization of water, with a special focus on the constant Kw.
- 💧 The concept of water acting as both an acid and a base (amphoteric behavior) is explored, detailing the self-ionization process of water and its equilibrium expression.
- 📈 The lecture introduces the relationship between pKw, pH, and pOH, explaining how these values interrelate and their significance at room temperature.
- 📊 The significance of pH in determining the acidity or alkalinity of a solution is covered, with examples of how different substances measure on the pH scale.
- 🧪 A practical demonstration using pH paper to measure the pH of various substances like ammonia, soda, vinegar, and a prescription medicine is described.
- 💊 The potential harm of corrosive medicines is highlighted through the personal anecdote of a child's experience with an iron supplement, emphasizing the importance of pH in medication safety.
- 🔑 The strength of acids and bases is discussed in terms of their ionization constants (Ka for acids and Kb for bases), and the concept of weak and strong acids and bases is explained.
- 📘 The lecture provides an overview of how to calculate pH in solutions involving weak acids and bases, including the use of equilibrium expressions and the assumption of small x values.
- 📚 The importance of understanding the relationship between significant figures in scientific calculations is stressed, especially in the context of pH and pOH values.
- 🚫 A warning is given about the potential dangers of not considering the corrosive nature of substances, especially in medical contexts, and the role of education in preventing such issues.
Q & A
What is the purpose of MIT OpenCourseWare and how can one support it?
-MIT OpenCourseWare aims to offer high-quality educational resources for free. Support can be provided through donations or by viewing additional materials from hundreds of MIT courses on their website, ocw.mit.edu.
What is a Lewis base and how does it interact with a Lewis acid?
-A Lewis base is a molecule that donates a lone pair of electrons. It interacts with a Lewis acid, which is a molecule that accepts these electrons, forming a bond between them.
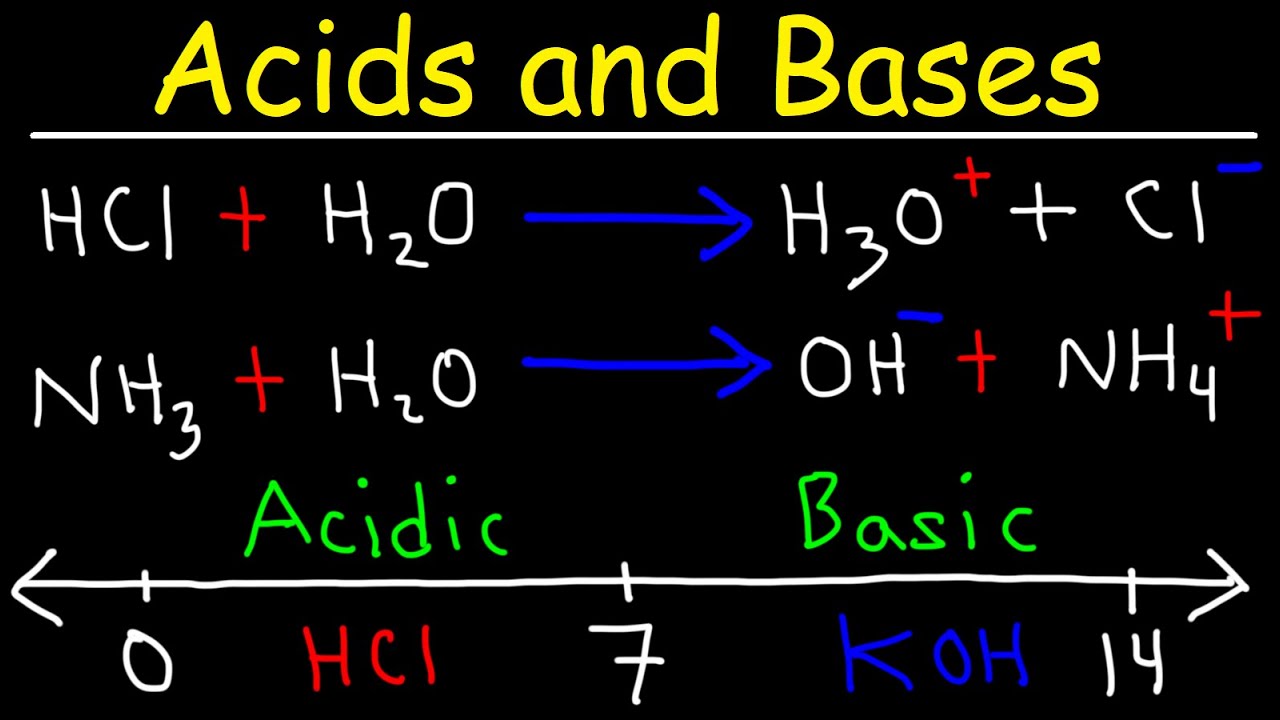
Can you explain the Bronsted-Lowry definition of acids and bases?
-According to the Bronsted-Lowry definition, an acid is a substance that donates a hydrogen ion (H+ or proton), while a base is a substance that accepts a hydrogen ion.
How are the Lewis and Bronsted-Lowry definitions related?
-The Lewis and Bronsted-Lowry definitions can be compatible and complementary. The Lewis definition focuses on the donation and acceptance of electron pairs, whereas the Bronsted-Lowry definition is centered around the hydrogen ion or proton transfer.
What is the significance of water in acid-base chemistry?
-Water is a crucial solvent in acid-base chemistry because it can act as both an acid and a base, undergoing self-ionization to form hydronium (H3O+) and hydroxide (OH-) ions.
What is the equilibrium constant for water (Kw) and how is it used in acid-base calculations?
-The equilibrium constant for water, Kw, is equal to the product of the concentrations of hydronium and hydroxide ions, which at room temperature is always 1.0 x 10^-14. It is used to relate the concentrations of these ions in acid-base equilibrium problems.
Define pH, pOH, and pKw, and explain their relationship.
-pH is the negative logarithm of the hydronium ion concentration, pOH is the negative logarithm of the hydroxide ion concentration, and pKw is the negative logarithm of the water ion product constant (Kw). The relationship between them is given by the equation pKw = pH + pOH, which equals 14 at room temperature.
How can one estimate the pH of a solution using pH paper?
-pH paper has an indicator that changes color depending on the pH of the solution it is dipped into. By comparing the color change to a reference chart, one can estimate the pH of the solution.
What is the difference between a strong acid and a weak acid in terms of their ionization in water?
-A strong acid ionizes almost completely in water, meaning the concentration of the acid in water is equal to the concentration of the resulting hydronium ions. A weak acid, on the other hand, only partially ionizes in water, resulting in a much lower concentration of hydronium ions compared to the initial acid concentration.
What is the acid ionization constant (Ka) and how does it relate to the strength of an acid?
-The acid ionization constant (Ka) is the equilibrium constant for the ionization of an acid in water. It indicates the extent to which an acid ionizes to form hydronium ions and its conjugate base. A higher Ka value signifies a stronger acid, as it ionizes more in solution.
Can you explain the concept of significant figures in the context of scientific calculations?
-Significant figures are the digits in a number that carry meaningful information about its precision. In scientific calculations, the number of significant figures in the final answer should match the number of significant figures in the given data or calculation steps, ensuring the accuracy and reliability of the result.
Outlines
📚 Introduction to MIT OpenCourseWare and Lewis Acid-Base Concepts
The script begins with an introduction to MIT OpenCourseWare, encouraging donations to support free educational resources, with a specific mention of the platform's website. It then transitions into a chemistry lecture, discussing a review of Lewis acid-base definitions where a Lewis base donates a lone pair of electrons to a Lewis acid. The lecture includes an interactive element with the audience, exploring the Bronsted-Lowry definitions of acids and bases and comparing them to the Lewis definitions. The discussion aims to show the compatibility between the two sets of definitions.
🔬 Continuation of Acid-Base Concepts and the Role of Water
This paragraph continues the chemistry lecture, focusing on the relationship between pKw, pH, and pOH, and the strengths of acids and bases. It introduces the concept of water as an amphoteric substance, capable of acting as both an acid and a base, forming H3O+ and OH- ions respectively. The lecturer explains the equilibrium constant (K) in the context of water and how it relates to the Gibbs free energy (delta G0), leading into a discussion about the spontaneity of the forward reaction and the calculation of K from delta G0.
💧 The Ionization of Water and its Equilibrium Constant (Kw)
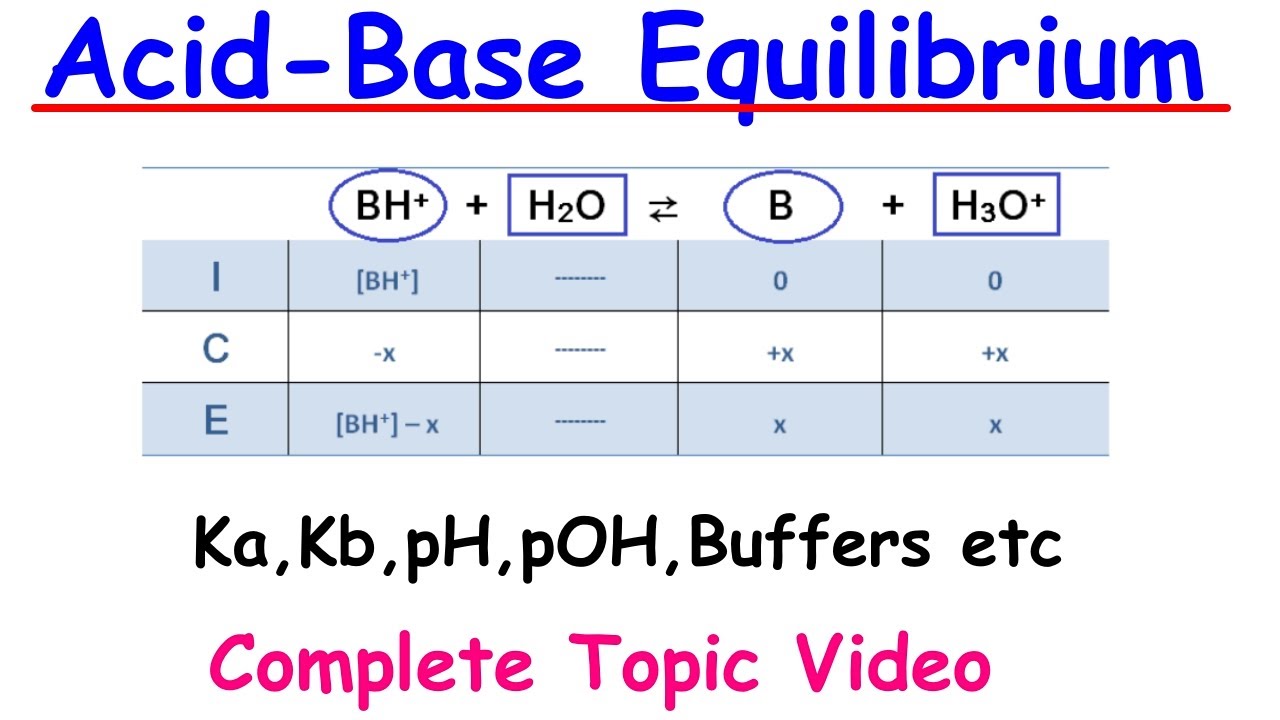
The lecturer delves into the ionization of water, explaining that only a small fraction of water molecules are ionized, with the equilibrium constant for water (Kw) being 1.0 x 10^-14 at room temperature. The importance of Kw in acid-base problems is emphasized, as it represents the product of the hydronium and hydroxide ion concentrations. The explanation includes the exclusion of pure solvents like water from equilibrium expressions and introduces the concepts of pH and pOH, relating them to the concentrations of H3O+ and OH- ions.
📈 pH, pOH, and the Relationship with pKw
This section discusses the relationship between pH, pOH, and pKw, highlighting the mathematical connection that pH + pOH = pKw = 14 at room temperature. The practical application of this relationship in problem-solving is emphasized, as is the significance of pH in determining the acidity or alkalinity of a solution. The lecture also mentions a problem set due on Friday, indicating that it will involve various acid-base problems.
🧪 Practical Demonstration of pH Measurement
The script describes a practical demonstration of pH measurement using pH paper to test various substances, including ammonia, soda, vinegar, tap water, and a prescription medicine. The audience is engaged by having volunteers measure the pH of these substances, emphasizing the importance of understanding pH in everyday life, especially in the context of medicine and its potential effects on health.
🚫 The Dangers of Corrosive Substances and the Importance of pH
The lecture discusses the corrosive nature of certain substances, with a pH less than 3 or greater than 12.5, as defined by the EPA. It uses the example of a medicine with a pH of 2, which was found to cause sores in the lecturer's daughter's mouth, to illustrate the importance of being aware of the pH of substances we consume or administer to others. The lecturer shares a personal anecdote about finding an alternative way to administer iron supplements to her daughter using Nutella to mask the taste of the medicine, reinforcing the significance of pH in healthcare.
🌡 Strength of Acids and the Ionization Constant (Ka)
The script explains the concept of the strength of acids, introducing the acid ionization constant (Ka) as a measure of how much an acid ionizes in solution. It contrasts strong acids, which have a high Ka and ionize almost completely, with weak acids that have a low Ka and ionize to a lesser extent. The lecture includes an example of acetic acid, discussing its ionization in water and the resulting formation of hydronium ions and the acetate ion.
🔑 The Significance of pKa in Acid Strength
This section introduces pKa as the negative logarithm of Ka, emphasizing its importance in various scientific fields, including organic chemistry and biology. The lecture clarifies the relationship between Ka and pKa, noting that a higher pKa indicates a weaker acid, and vice versa. The importance of understanding pKa is highlighted, with a humorous anecdote about students in organic chemistry being unaware of the concept.
📉 Comparing Acid Strengths and the Concept of Conjugate Acids and Bases
The script compares the strengths of different acids using their Ka and pKa values, illustrating the concept with examples from a table of acids. It explains that a strong acid will have a Ka much greater than 1 and a very low pKa, while a weak acid will have a Ka less than 1 and a higher pKa. The lecture also introduces the concept of conjugate acids and bases, noting that the strength of an acid is inversely related to the strength of its conjugate base.
📚 Summary of Acid and Base Concepts and预告of Buffers
The script summarizes the key concepts discussed in the lecture, including the strength of acids and bases, Ka and Kb values, and the relationship between conjugate acids and bases. It previews the topic of buffers, which will be discussed in a future class, and emphasizes the importance of understanding these concepts for future applications in various scientific fields.
🧪 Calculation of pH for a Weak Acid Solution
This section describes the process of calculating the pH of a weak acid solution, using vitamin C as an example. The lecture explains the steps for converting mass to moles, calculating molarity, and setting up an equilibrium expression with the given Ka value. It discusses the assumption that the change in concentration (x) is small, allowing for simplification of the quadratic equation, and emphasizes the importance of checking this assumption for accuracy.
🔢 Detailed Steps for Calculating pH of a Weak Base Solution
The script outlines the detailed steps for calculating the pH of a weak base solution, such as ammonia in water. It explains how to set up an equilibrium table, write the Kb expression, and calculate the change in molarity. The lecture demonstrates the use of the assumption that x is small to simplify calculations and the importance of checking this assumption. It concludes with the calculation of pOH and then pH, ensuring that the final pH value makes sense for a basic solution.
Mindmap
Keywords
💡Creative Commons license
💡MIT OpenCourseWare
💡Lewis base
💡Lewis acid
💡Bronsted-Lowry definition
💡Amphoteric
💡Equilibrium constant (K)
💡pKw, pH, and pOH
💡Acid ionization constant (Ka)
💡Base ionization constant (Kb)
💡pH paper
💡Conjugate acid-base pair
Highlights
Introduction to MIT OpenCourseWare and its commitment to providing free educational resources.
Review of Lewis acid-base definitions and their importance in understanding chemical interactions.
Explanation of the Bronsted-Lowry definition and its comparison with the Lewis definition.
Discussion on the compatibility of Lewis and Bronsted-Lowry definitions in acid-base chemistry.
Illustration of how water can act as both an acid and a base, emphasizing its amphoteric nature.
Introduction to the concept of equilibrium constants and their role in acid-base reactions.
Calculation of the equilibrium constant (Kw) for water and its significance at room temperature.
Explanation of the relationship between pH, pOH, and pKw in aqueous solutions.
Demonstration of measuring pH levels using pH paper and its practical applications.
Analysis of the pH levels of various substances, including water, soda, vinegar, and a prescription medicine.
Importance of pH in determining the corrosiveness of a substance and its implications for health.
Introduction to the strength of acids and the concept of the acid ionization constant (Ka).
Differentiation between strong and weak acids based on their Ka values and extent of ionization.
Introduction to pKa and its significance in various scientific fields, including organic chemistry.
Discussion on the relationship between Ka, pKa, and the strength of an acid.
Overview of the process for calculating the pH of a weak acid solution using its Ka value.
Emphasis on the importance of significant figures in scientific calculations and reporting.
Advice on approaching problem sets and exams involving acid-base chemistry, including time management and checking answers.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: