12.1 Introduction to Acids and Bases | High School Chemistry
TLDRThis chemistry lesson introduces acids and bases, covering definitions, properties, and the distinction between strong and weak acids and bases. It explains the Arrhenius and Brønsted-Lowry definitions, discusses litmus paper, and highlights the importance of not tasting or touching acids and bases in the lab. The lesson also touches on conjugate acid-base pairs and amphiprotic substances, setting the stage for upcoming lessons on pH calculations, buffers, and titrations.
Takeaways
- 📚 The lesson is an introduction to acids and bases, part of a larger chapter on the topic, and includes definitions, properties, and discussions on strong acids, bases, salts, and how they interact.
- 🔍 Acids are associated with the color red and bases with blue, a convention used in laboratories to distinguish between them using litmus paper.
- 🍋 Acids have a sour taste, while bases are bitter, but they should never be tasted in a laboratory setting due to their corrosive nature.
- 🚫 Bases can be slippery and are also caustic, so they should not be touched in a lab environment.
- 🔬 The Arrhenius definition of acids and bases is based on the donation of H+ ions (protons) in water for acids and OH- ions for bases.
- 🌐 The Bronsted-Lowry definition expands on the Arrhenius definition, stating that acids are proton donors and bases are proton acceptors, applicable in any solvent, not just water.
- 🔄 In acid-base reactions, the acid donates a proton and becomes its conjugate base, while the base accepts a proton and becomes its conjugate acid.
- 🔄 Amphiprotic substances, like water, can act as both acids and bases, donating or accepting protons depending on the reaction.
- 💧 Strong acids and bases are strong electrolytes that dissociate completely in water, such as HCl and NaOH, while weak acids and bases do not dissociate completely.
- 🧪 Polyprotic acids like H2SO4 can donate multiple protons, but only the first dissociation is considered strong, making it a diprotic strong acid.
- 📈 The solubility of group 1 and group 2 metal hydroxides in water is high, making them strong bases, but their actual solubility can vary, affecting their classification.
Q & A
What is the main topic of this lesson?
-The main topic of this lesson is an introduction to acids and bases, including definitions, properties, and the distinction between strong acids, bases, and salts.
How can you distinguish between an acid and a base using litmus paper?
-Litmus paper is a color-changing indicator. When an acid is poured on it, it turns red, and when a base is poured on it, it turns blue.
What is the Arrhenius definition of an acid?
-According to the Arrhenius definition, an acid is a substance that donates hydrogen ions (H+) in water.
What is the Arrhenius definition of a base?
-According to the Arrhenius definition, a base is a substance that donates hydroxide ions (OH-) in water.
What is the difference between an Arrhenius acid-base reaction and a Bronsted-Lowry acid-base reaction?
-An Arrhenius acid-base reaction always results in the formation of water and a salt, while a Bronsted-Lowry acid-base reaction does not necessarily form water and can occur in any solvent, not just water.
What is the relationship between an acid and its conjugate base in a Bronsted-Lowry acid-base reaction?
-In a Bronsted-Lowry acid-base reaction, an acid that donates a hydrogen ion (H+) becomes its conjugate base, and a base that accepts a hydrogen ion becomes its conjugate acid.
What is an amphiprotic substance?
-An amphiprotic substance is one that can act as either a proton donor (acid) or a proton acceptor (base), such as water.
Why are strong acids and bases considered strong electrolytes?
-Strong acids and bases are considered strong electrolytes because they dissociate completely into ions in water, donating or accepting protons fully.
What is the difference between monoprotic and polyprotic acids?
-Monoprotic acids, like HCl, donate one proton (H+) in water, while polyprotic acids, like H2SO4, can donate multiple protons. H2SO4, for example, is diprotic and can donate two protons, but only the first dissociation is complete in a strong acid manner.
How do you identify a weak acid from its chemical formula?
-A weak acid is identified by its chemical formula starting with an 'H' and not being one of the seven strong acids listed in the study guide. If it starts with an 'H' and is not a strong acid, it is a weak acid.
What are some examples of strong bases mentioned in the script?
-Examples of strong bases mentioned in the script include sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)2), and barium hydroxide (Ba(OH)2). These are all metal hydroxides from group 1 and group 2 metals.
Outlines
🔬 Introduction to Acids and Bases
This paragraph introduces the topic of acids and bases, marking it as the first lesson in a comprehensive chapter on the subject. The speaker outlines the plan for the series, which includes discussions on definitions, strong acids and bases, salts, pH calculations, and finally, buffers and titrations. The lesson is part of a high school chemistry playlist, with new lessons released weekly. The speaker also humorously recounts a mishap with recording equipment, highlighting the importance of attention to detail in both chemistry and video production. The paragraph concludes with a basic definition of acids and bases, emphasizing their properties such as color change with litmus paper, taste, and physical sensations like slipperiness.
🧪 Definitions and Properties of Acids and Bases
The speaker delves into the definitions of acids and bases according to Arrhenius and Brønsted-Lowry theories. Acids are described as substances that donate hydrogen ions (H+) in water, with a focus on the common example of hydrochloric acid (HCl). The concept of hydronium ions (H3O+) is introduced, explaining the reaction of H+ with water molecules. Bases, on the other hand, are defined as substances that donate hydroxide ions (OH-) in water, with sodium hydroxide (NaOH) serving as a typical example. The paragraph also covers the reaction between acids and bases, resulting in water and a salt, and the broader definition of acids and bases in Brønsted-Lowry theory, which includes reactions not limited to aqueous solutions.
🔄 Brønsted-Lowry Acid-Base Reactions and Equilibrium
This paragraph further explores the Brønsted-Lowry definition of acids and bases, focusing on the concept of equilibrium in acid-base reactions. The speaker uses the example of hydrochloric acid (HCl) reacting with ammonia (NH3) to illustrate how an acid can donate a proton (H+) and a base can accept it. The dynamic equilibrium of such reactions is emphasized, where the forward and reverse reactions occur simultaneously. The idea of conjugate acid-base pairs is introduced, explaining how an acid can become a base and vice versa in the context of a reaction. The paragraph also touches on the relationship between the strength of an acid and the weakness of its conjugate base, and vice versa.
🌊 Amphiprotic Species and Strong Acids and Bases
The concept of amphiprotic species, which can act as both acids and bases, is introduced with water as an example. Water can donate a proton to act as an acid or accept a proton to act as a base. The paragraph then discusses strong acids and bases, emphasizing their complete dissociation in water. Lists of strong acids and bases are provided, with a focus on their electrolytic properties. The distinction between monoprotic and polyprotic acids is made, using sulfuric acid (H2SO4) as an example of a diprotic acid. The paragraph concludes with a brief mention of the solubility and dissociation of group 1 and group 2 metal hydroxides, which are considered strong bases.
📚 Recap and Further Learning Resources
In the final paragraph, the speaker summarizes the key points discussed in the lesson, including the definitions of acids and bases, the properties of strong acids and bases, and the concept of amphiprotic species. The speaker encourages viewers to like and share the video to help others find the content. Additionally, a study guide and practice problems related to the lesson are mentioned, with a promotion for a premium course available on ChatsPrep.com, offering a free trial for interested learners.
Mindmap
Keywords
💡Acid
💡Base
💡Litmus Paper
💡pH Scale
💡Salt
💡Strong Acid
💡Strong Base
💡Weak Acid
💡Conjugate Acid-Base Pair
💡Amphoteric
💡Polyprotic Acid
Highlights
Introduction to the chemistry of acids and bases, including definitions and properties.
Explanation of the color change with litmus paper as an indicator for acids and bases.
Taste properties of acids and bases, with warnings against tasting lab chemicals.
Definition of acids and bases according to Arrhenius, including H+ donor and OH- donor concepts.
Discussion on the representation of hydrogen ions in water as H3O+ instead of H+.
Introduction of the Bronsted-Lowry definition of acids and bases as proton donors and acceptors.
Explanation of conjugate acid-base pairs and their relationship in reactions.
The concept of amphiprotic species, such as water, which can act as both an acid and a base.
Differentiation between strong and weak acids and bases based on their degree of dissociation in water.
List of seven strong acids that are important to memorize for chemistry studies.
Description of strong bases, particularly metal hydroxides, and their complete dissociation in water.
Clarification on the term 'strong electrolyte' and its relation to strong acids and bases.
Discussion on polyprotic acids like H2SO4 and their dissociation behavior in water.
Differentiation between monoprotic and polyprotic acids based on the number of protons they can donate.
Note on the solubility and dissociation of group 2 metal hydroxides as strong bases.
The inverse relationship between the strength of an acid and its conjugate base.
Encouragement for viewers to like, share, and subscribe for weekly chemistry lessons.
Mention of a premium course on ChatsPrep.com for further study and practice.
Transcripts
Browse More Related Video

16.1 Introduction to Acids and Bases | General Chemistry
Acid Base Equilibrium Full Topic Video

3.1 Introduction to Acids and Bases | Organic Chemistry

21. Acid-Base Equilibrium: Is MIT Water Safe to Drink?

Introduction to Acid-Base Chemistry - AP Chemistry Unit 4, Topic 8
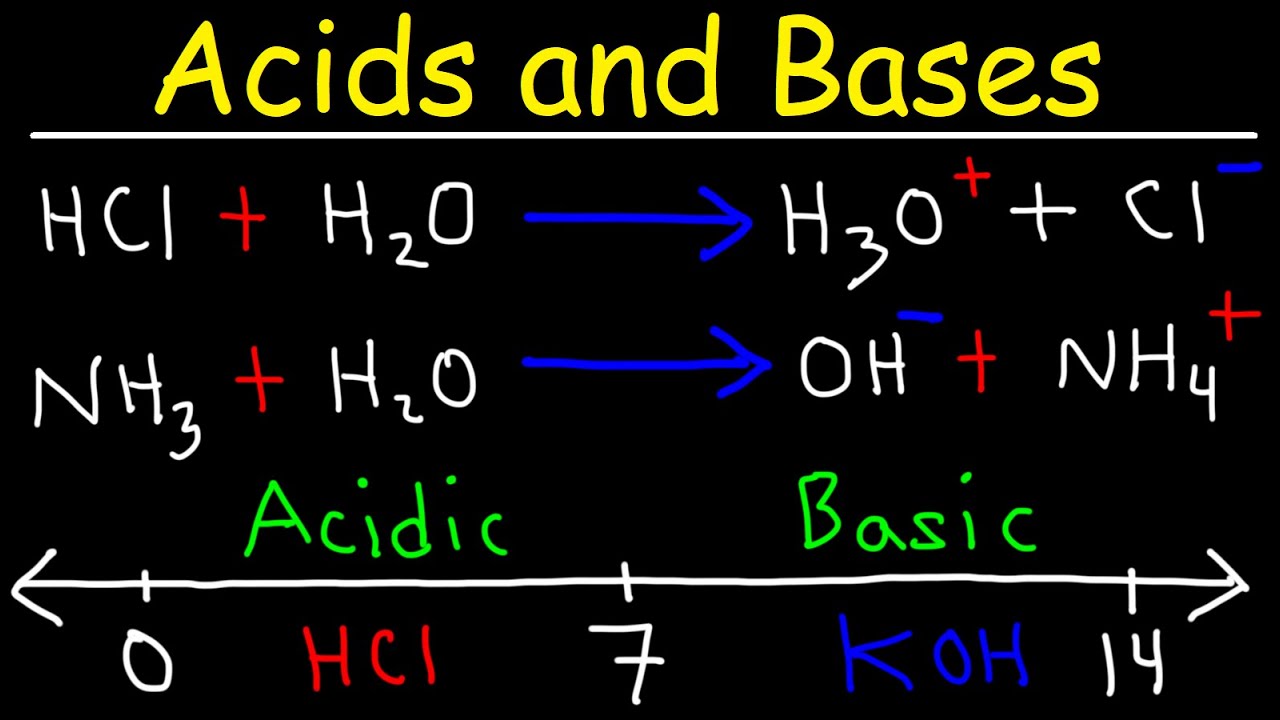
Acids and Bases - Basic Introduction - Chemistry
5.0 / 5 (0 votes)
Thanks for rating: