Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (10 of 92) What is Normalization? Ex. 1
TLDRIn this video, the concept of normalization in quantum mechanics is explored to determine the probability of finding a particle at a specific location. The script explains that the probability density function, or wave function, must be normalized so that its integral over all possible locations equals 1, representing a 100% probability. The process involves finding the correct constant to normalize the wave function, ensuring that the probability density accurately reflects the true likelihood of the particle's presence at any given point within a defined range.
Takeaways
- 📈 The concept of a probability density function (PDF) is introduced to describe the likelihood of finding a particle at a specific location.
- 🌐 Normalization of the PDF is necessary to ensure that the integral of the function over all possible locations equals 1, representing a 100% probability.
- 📌 The amplitude of the PDF indicates the relative likelihood of finding a particle at a particular location, but it is not an absolute probability.
- 🔄 The product of a wave function and its complex conjugate results in a real number, which is crucial for normalization.
- 🧬 For a particle confined between points A and B, the wave function must be normalized such that the integral from A to B equals 1.
- 🔢 The constant 'a' in the wave function is determined by the condition that the integral from A to B equals 1, leading to 'a' being equal to 1 over the square root of (B - A).
- 🌊 The normalized wave function for a single particle in one dimension with no forces acting on it and constant potential energy is given by 1 over the square root of (B - A) times e to the (±IKX - ΩT).
- 🎲 The normalization process ensures that the probability density function accurately represents the true probability of finding a particle at any given location.
- 📊 The area under the curve of the PDF represents the probability of finding the particle within a certain range of positions.
- 🔍 By selecting a value for X, one can determine the exact probability of finding the particle at that specific location.
- 📈 Normalization is a fundamental step in quantum mechanics to ensure that the wave function represents a physically meaningful probability distribution.
Q & A
What is the purpose of normalizing the probability density function?
-The purpose of normalizing the probability density function is to ensure that the integral of the function over the entire possible range of values equals 1, which represents a 100% probability of finding the particle within that range.
What does a large amplitude in the probability density function indicate?
-A large amplitude in the probability density function indicates a higher likelihood of finding the particle at that particular location.
What does a small amplitude in the probability density function indicate?
-A small amplitude in the probability density function indicates a lower likelihood of finding the particle at that particular location.
What is the condition that must be satisfied for a function to be considered a valid probability function?
-The condition that must be satisfied for a function to be considered a valid probability function is that the integral of the product of the wave function and its complex conjugate over all space must equal one.
Outlines
🌟 Introduction to Probability Density Function
This paragraph introduces the concept of a probability density function (PDF) and its importance in quantum mechanics. It explains that the PDF, denoted as P(X, T), describes the likelihood of finding a particle at a specific location 'X'. The amplitude of the PDF indicates the relative probability, with larger amplitudes suggesting higher likelihood and vice versa. However, it emphasizes that the probability is relative and not an absolute value, as the area under the curve of the PDF must equal 1, representing a 100% probability over its defined range. The paragraph also sets up a hypothetical scenario where a particle is confined between 'a' and 'B', and discusses the need to normalize the wave function to ensure the total probability is 1 within this range.
📏 Normalization of the Wave Function
This paragraph delves into the process of normalizing the wave function to obtain a probability density function. It explains that normalization involves finding the correct constant 'a' such that the integral of the product of the wave function and its complex conjugate over the entire possible range of the particle's location equals 1. This ensures that the probability density function accurately represents the true probability of finding the particle at any given location. The paragraph provides a mathematical example of normalizing a wave function for a single particle in one dimension with no forces acting on it and a constant potential energy. The resulting normalized wave function is given as 1/√(B - a) times e^(i(kx - ωt)), highlighting the significance of the normalization process in quantum mechanics.
Mindmap
Keywords
💡Normalization
💡Probability Density Function (PDF)
💡Wave Function
💡Complex Conjugate
💡Integral
💡Amplitude
💡Quantum Mechanics
💡Potential Energy
💡One-Dimensional
💡Constant Potential Energy
💡Particle
Highlights
The importance of normalizing the probability density function to ensure the total probability equals 1.
The concept of probability density and how it relates to finding a particle at a particular location.
The equation P(X,T) as the probability distribution function for a particle's location.
The condition that the integral of the wave function and its complex conjugate over all space must equal 1.
The hypothetical scenario of a particle being more likely to be found in the middle than close to the edges (between points A and B).
The mathematical process of normalizing the wave function by integrating from A to B and ensuring the result equals 1.
The product of the wave function and its complex conjugate resulting in a squared term, which simplifies to 1.
The method for finding the constant 'a' by integrating from A to B and setting the result equal to 1/(B-A).
The final normalized wave function for a single particle with no forces acting on it and constant potential energy.
The significance of the constant 'a' being equal to 1/√(B-A) for the wave function to be normalized.
The practical application of the normalized wave function in determining the true probability of finding a particle at a specific location.
The process of integrating the product of the wave function and its complex conjugate over a defined range.
The role of the complex conjugate in the normalization process of the wave function.
The concept that the area under the probability density function curve represents the probability of finding a particle within a certain range.
The explanation of how the wave function's constant is determined to ensure the probability density function is normalized.
The discussion of how the normalized wave function allows for the direct reading of the probability of finding a particle at a specific location.
Transcripts
Browse More Related Video
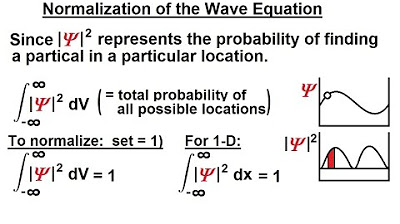
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (2 of 92) Normalization of the Wave Equation
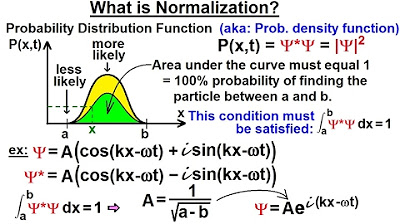
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (11 of 92) What is Normalization? Ex. 2
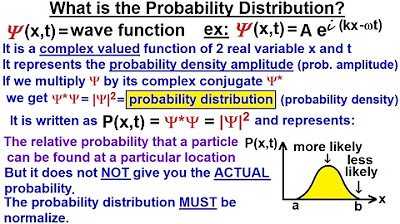
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (9 of 92) What is the Prob Distribution?
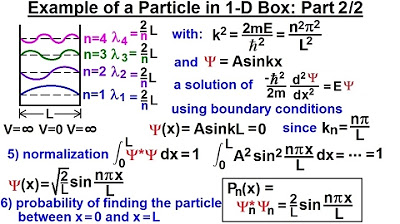
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (20 of 92) Particle in 1-D Box: Example 2/2

Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (25 of 92) Prob. of a Particle 1-D Box n=1
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (18 of 92) Particle in 1-D Box: Gen. Appr.
5.0 / 5 (0 votes)
Thanks for rating: