Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (25 of 92) Prob. of a Particle 1-D Box n=1
TLDRThis video delves into the probability of locating a particle within a specific section of a one-dimensional box, depending on its energy state. The ground state probability density function resembles a sine or cosine wave, with the highest likelihood in the box's center. In contrast, for the first excited state (N=2), the probability at the box's midpoint is zero. The video illustrates this by calculating the probability density function for the ground state and shows that the particle's probability distribution changes significantly with its energy level, affecting the likelihood of finding it in a particular box region.
Takeaways
- 🌟 The probability of finding a particle in a one-dimensional box depends on its energy state, with different patterns for ground state and excited states.
- 📈 In the ground state (N=1), the probability density function resembles a sine or cosine wave, with the highest probability in the middle of the box.
- 📊 For the first excited state (N=2), the probability density function changes significantly, with the probability at the box's center being zero.
- 🧪 The video demonstrates the calculation of the probability density function for the ground state using the Schrödinger equation and integrating over a specific range within the box.
- 🔢 By integrating the probability density function from 0.45L to 0.55L, the video shows that there's a 19.8% chance of finding the particle in that range, despite it being only 10% of the box's length.
- 🌐 The probability distribution is not uniform across the box, with certain regions having a higher likelihood of finding the particle based on its energy state.
- 🎥 The video script includes a step-by-step mathematical breakdown of the integrals involved in calculating the probability.
- 📚 The use of trigonometric identities and proper differentials is crucial for accurate integration and probability calculation.
- 🔄 The process of splitting the integral into separate parts and evaluating them individually is highlighted to simplify the calculation.
- 📈 The results indicate that the particle is more likely to be found in certain areas of the box, which aligns with the wave-like nature of the probability density function.
- 🚀 The video sets up for a follow-up exploration in the next episode, where the probability for the particle in the second energy level will be examined.
Q & A
What is the main topic of the video?
-The main topic of the video is exploring the probability of finding a particle in a particular portion of a one-dimensional box, depending on its energy state.
How does the probability density function change with the energy state of the particle?
-The probability density function changes significantly with the energy state of the particle. At the ground state, it resembles a sine or cosine wave, with the highest probability in the middle of the box. However, at the first excited state (second energy level), the probability at the center of the box goes to zero.
What is the mathematical equation used to describe the position of a particle in a box?
-The position of a particle in a box is described by a surging equation, which is modified by the energy level indicator 'n' at the end of the equation.
How is the probability density function derived for the ground state?
-For the ground state (n=1), the probability density function is derived by squaring the function obtained from the surging equation.
What is the process to calculate the probability of finding a particle within a specific region in the box?
-To calculate the probability, one integrates the probability density function from the boundaries X1 to X2 of the region of interest within the box.
What is the significance of the integral limits 0.45L and 0.55L?
-The integral limits 0.45L and 0.55L are chosen to symmetrically straddle the central point of the box, allowing for a more meaningful comparison of probabilities across different energy states.
How does the probability of finding a particle in a specific region compare between the ground state and the first excited state?
-In the ground state, there is a higher probability of finding the particle in the middle of the box, whereas in the first excited state, the probability at the center is zero, indicating a significantly different distribution.
What is the final calculated probability of finding the particle between 0.45L and 0.55L?
-The final calculated probability of finding the particle between 0.45L and 0.55L is 19.8%, despite this range being only 10% of the total distance from 0 to L.
What is the implication of the calculated probability for the understanding of particle behavior in a one-dimensional box?
-The calculated probability enhances our understanding that the behavior and location of a particle in a one-dimensional box are highly dependent on its energy state, with significant variations in the probability density function from one state to another.
What will be the focus of the next video?
-The next video will continue the exploration of particle probability in a one-dimensional box, but this time for the second energy level, to further illustrate how the probability changes with different energy states.
Outlines
🌟 Introduction to Probability in a One-Dimensional Box
This paragraph introduces the concept of finding the probability of a particle's presence in a specific section of a one-dimensional box, emphasizing how this probability varies with the particle's energy state. It explains that at the ground state, the probability density function resembles a sine or cosine wave, with the highest probability in the middle of the box. In contrast, for the first excited state (N=2), the probability at the box's center is zero. The video aims to calculate the probability for these scenarios, starting with the ground state and using the particle in a box model to derive the probability density function. The process involves squaring the wave function and integrating over the region of interest to find the probability of the particle's presence.
📈 Calculating Probability for Ground State and First Excited State
This paragraph delves into the mathematical process of calculating the probability of finding a particle in a one-dimensional box for both the ground state and the first excited state. It describes the integration of the probability density function, which is derived from the wave function, over a symmetrical range around the box's center. The calculation involves splitting the integral into two parts and simplifying the expression using trigonometric identities. The result shows that there is a 19.8% chance of finding the particle in a range that represents only 10% of the total length of the box, which is a higher probability than if the particle were distributed evenly. The paragraph concludes by noting that the probability will be significantly different for the second energy level, which will be explored in the next video.
Mindmap
Keywords
💡Probability
💡Particle
💡One-Dimensional Box
💡Energy State
💡Ground State
💡Excited State
💡Probability Density Function
💡Quantum Mechanics
💡Wave Function
💡Schrödinger Equation
💡Integration
Highlights
Exploration of probability of finding a particle in a specific portion of a one-dimensional box.
The probability depends on the particle's state, specifically its energy level.
At the ground state, the probability density function resembles a sine or cosine wave.
Highest probability at the ground state is found in the middle of the box.
For the first excited state (N=2), the probability density function looks significantly different.
Probability at the center of the box goes to zero for the first excited state.
Calculation of the probability density function for the ground state is demonstrated.
The Schrödinger equation is used to describe the position of a particle in a box.
For the ground state (n=1), squaring the function of the Schrödinger equation yields the probability density function.
Integration of the probability density function over specific boundaries gives the probability of finding the particle.
The integral is split into two parts for simplification, involving the sine and cosine of a specific angle.
The final result shows a 19.8% probability of finding the particle in a 10% section of the box length.
The probability is not evenly distributed across the box, with a higher chance in certain areas.
The video will continue to explore the probability for particles in higher energy states in future content.
The particle's probability distribution in a one-dimensional box varies greatly with its energy state.
The practical application of these findings can help understand quantum behavior in particles.
The method used can be applied to find probabilities in three-dimensional boxes as well.
The video provides a clear and detailed explanation of the mathematical process involved in finding these probabilities.
Transcripts
Browse More Related Video

Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (27 of 92) Expectation Value=? 1-D Box n=1
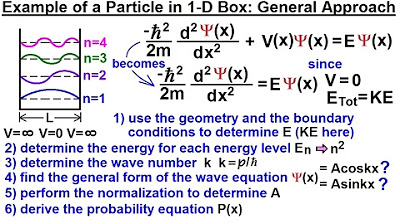
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (18 of 92) Particle in 1-D Box: Gen. Appr.
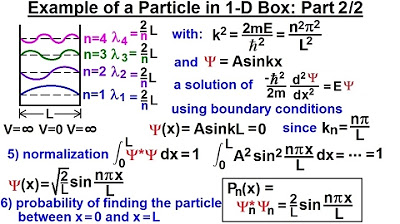
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (20 of 92) Particle in 1-D Box: Example 2/2

Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (10 of 92) What is Normalization? Ex. 1
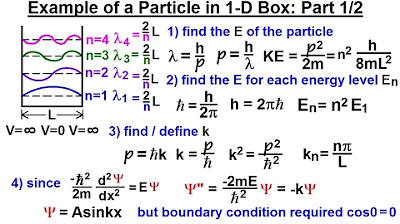
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (19 of 92) Particle in 1-D Box: Example 1/2
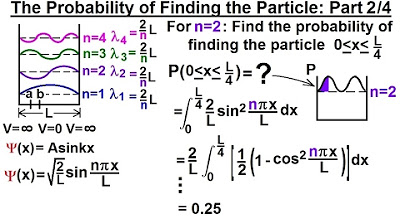
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (22 of 92) Prob. of Finding Particle 2
5.0 / 5 (0 votes)
Thanks for rating: