Daltons Law | Partial Pressures
TLDRIn this educational video, Dr. Mike explains Dalton's Law, a fundamental principle in understanding respiratory physiology, including diving and aviation. He clarifies that the total atmospheric pressure is the sum of the partial pressures of individual gases, such as nitrogen (78.6%), oxygen (20.9%), and carbon dioxide (0.04%). By illustrating the concept with the example of a syringe, he demonstrates how increased pressure affects gas concentrations, which is crucial for comprehending gas exchange according to Henry's Law.
Takeaways
- 📚 Dalton's Law is essential for understanding respiratory physiology, including diving and aviation physiology.
- 🌐 Dalton's Law states that the total atmospheric pressure is the sum of the partial pressures of individual gases.
- 🌡 At sea level, the total atmospheric pressure is 760 millimeters of mercury.
- 🌌 The atmosphere's composition includes nitrogen (N2) at 78.6%, oxygen (O2) at 20.9%, carbon dioxide (CO2) at 0.04%, and trace gases.
- 📊 Partial pressures can be calculated by multiplying the percentage of each gas by the total atmospheric pressure (e.g., nitrogen's partial pressure is 597 mmHg).
- 🔍 Boyle's Law is related to Dalton's Law, explaining the inverse relationship between the volume of a container and the pressure within it.
- 🌊 When diving deeper, the atmospheric pressure increases, which affects the partial pressures of gases like nitrogen, oxygen, and carbon dioxide.
- 💡 The increase in total pressure, such as during deep diving, results in an increase in the partial pressures of the individual gases.
- 🤿 Henry's Law is mentioned in relation to Dalton's Law, highlighting the importance of partial pressures in gas exchange processes.
- 🔄 Dalton's Law helps us understand how the concentration or pressure of gases changes with changes in total atmospheric pressure.
Q & A
What is Dalton's Law and why is it important for understanding respiratory physiology?
-Dalton's Law states that the total atmospheric pressure is the sum of the partial pressures of individual gases. It's important for understanding respiratory physiology because it helps explain how different gases in the atmosphere contribute to the overall pressure we experience, which is crucial for processes like breathing, diving, and aviation.
What is the total atmospheric pressure at sea level in millimeters of mercury?
-The total atmospheric pressure at sea level is 760 millimeters of mercury.
What does it mean to say that the atmosphere is composed of individual gases exerting partial pressures?
-It means that each gas in the atmosphere contributes to the total atmospheric pressure to a degree proportional to its concentration. For example, nitrogen contributes the most due to its high concentration, followed by oxygen, carbon dioxide, and trace gases.
What percentage of the atmospheric gas is nitrogen (N2) and what is its partial pressure in millimeters of mercury?
-Nitrogen makes up 78.6% of the atmospheric gas, and its partial pressure is 597 millimeters of mercury.
How much does oxygen (O2) contribute to the total atmospheric pressure as a percentage and what is its partial pressure?
-Oxygen makes up 20.9% of the atmospheric pressure, and its partial pressure is approximately 159 millimeters of mercury.
What is the contribution of carbon dioxide (CO2) to the total atmospheric pressure and its partial pressure?
-Carbon dioxide makes up about 0.04% of the atmosphere, and its partial pressure is around 0.3 millimeters of mercury.
Why is the concept of partial pressure important in the context of diving or aviation?
-The concept of partial pressure is important in diving or aviation because it affects gas exchange in the body. As you go deeper in water or fly at higher altitudes, the total pressure increases, which in turn affects the partial pressures of the gases, impacting the body's ability to exchange gases efficiently.
How does Boyle's Law relate to the changes in atmospheric pressure when diving deeper underwater?
-Boyle's Law states that there is an inverse relationship between the volume of a container and the pressure of the container. When diving deeper, the volume of the space containing the air is reduced, which according to Boyle's Law, increases the pressure. This is why the total atmospheric pressure increases underwater, doubling for every 10 meters of descent approximately.
What is Henry's Law and how does it relate to Dalton's Law in the context of gas exchange?
-Henry's Law states that the amount of a given gas that dissolves in a liquid is directly proportional to the partial pressure of the gas in equilibrium with the liquid. It relates to Dalton's Law in that the partial pressures calculated by Dalton's Law determine how much of each gas will dissolve in the blood during gas exchange, which is vital for understanding respiratory processes.
Why might we not feel the atmospheric pressure even though it is 760 millimeters of mercury?
-We do not feel the atmospheric pressure of 760 millimeters of mercury because we are born into it and our bodies have adapted to this pressure. It is only when the pressure changes, such as during deep diving or in high-altitude aviation, that we might experience its effects.
How does the increase in total atmospheric pressure affect the partial pressures of individual gases?
-When the total atmospheric pressure increases, the partial pressures of individual gases also increase proportionally. For example, if the total pressure doubles, the partial pressure of nitrogen, which is 78.6% of the atmosphere, will also double from 597 to approximately 1194 millimeters of mercury.
Outlines
🌪️ Dalton's Law of Partial Pressures Explained
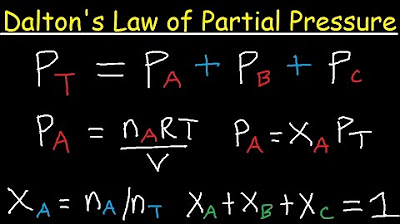
In this paragraph, Dr. Mike introduces Dalton's Law, a fundamental concept in understanding respiratory physiology, including diving and aviation. Dalton's Law states that the total atmospheric pressure is the sum of the partial pressures of the individual gases present. At sea level, the total atmospheric pressure is 760 millimeters of mercury, which is the result of adding the partial pressures of nitrogen (78.6%), oxygen (20.9%), carbon dioxide (0.04%), and trace gases. The partial pressures are calculated by applying the percentage of each gas to the total atmospheric pressure, resulting in 597 mmHg for nitrogen, approximately 159 mmHg for oxygen, and about 0.3 mmHg for carbon dioxide. The concept is crucial for understanding gas exchange in the body, as it affects how gases are absorbed and released in different environments.
🌊 Boyle's Law and the Impact on Partial Pressures
This paragraph delves into the relationship between Boyle's Law and Dalton's Law during scenarios like deep diving. Boyle's Law explains the inverse relationship between the volume of a gas and its pressure. When the volume of a container is decreased, the pressure increases, as demonstrated by pushing down on a plunger in a syringe, effectively doubling the atmospheric pressure from one to two atmospheres. This increase in pressure, according to Dalton's Law, means that the partial pressures of nitrogen, oxygen, and carbon dioxide also double. The partial pressure of nitrogen, for example, would be 78.6% of 1520 mmHg, effectively doubling its concentration or pressure. This understanding is vital for comprehending gas exchange as explained by Henry's Law, which is crucial in the context of diving or other high-pressure environments.
Mindmap
Keywords
💡Dalton's Law
💡Respiration
💡Atmospheric Pressure
💡Partial Pressure
💡Nitrogen (N2)
💡Oxygen (O2)
💡Carbon Dioxide (CO2)
💡Trace Gases
💡Boyle's Law
💡Henry's Law
💡Diving Physiology
Highlights
Introduction to Dalton's Law and its importance in understanding respiratory physiology.
Dalton's Law states that the total atmospheric pressure is the sum of the partial pressures of individual gases.
At sea level, the total atmospheric pressure is 760 millimeters of mercury.
Nitrogen (N2) is the most abundant gas in the atmosphere, making up 78.6% of the total atmospheric gas.
Oxygen (O2) accounts for 20.9% of the total atmospheric pressure.
Carbon dioxide (CO2) makes up a small 0.04% of the atmospheric pressure.
Trace gases contribute a negligible amount to the atmospheric pressure.
The partial pressure of nitrogen is calculated as 78.6% of 760 millimeters of mercury, equaling 597 millimeters of mercury.
The partial pressure of oxygen is approximately 159 millimeters of mercury.
The partial pressure of carbon dioxide is about 0.3 millimeters of mercury.
Understanding partial pressures is crucial for diving, aviation, and respiratory physiology.
Boyle's Law is mentioned, explaining the inverse relationship between the volume of a container and the pressure.
Increasing atmospheric pressure, such as in deep diving, affects the partial pressures of gases.
Doubling the atmospheric pressure doubles the partial pressures of nitrogen, oxygen, and carbon dioxide.
The relevance of Dalton's Law in understanding gas exchange is highlighted, with a reference to Henry's Law.
Henry's Law discusses gas exchange in relation to partial pressures, which is important in respiratory physiology.
Transcripts
Browse More Related Video
Dalton's Law of Partial Pressure Problems & Examples - Chemistry

Introduction to partial pressure | Gases and kinetic molecular theory | Chemistry | Khan Academy

9.3 Additional Gas Laws | Dalton's Law and Graham's Law | High School Chemistry

Partial pressure example | Chemistry | Khan Academy

Pressure and Gas Solubility (Henry's Law)

Henry's Law | Henry's Law Constant | Henry's Law Numericals
5.0 / 5 (0 votes)
Thanks for rating: