Partial pressure example | Chemistry | Khan Academy
TLDRThis script explores the calculation of total and partial pressures in a container filled with a mixture of gases (oxygen, hydrogen, and nitrogen). It demonstrates how to determine the moles of each gas, calculate the total pressure using the ideal gas law, and find the partial pressures of individual gases, emphasizing the importance of moles over mass in these calculations.
Takeaways
- 🎈 The scenario involves a four-meter cubed container filled with a mixture of oxygen, hydrogen, and nitrogen gases.
- 📊 The total mass of the gas mixture is 2.1 kilograms, with oxygen constituting 30.48%, hydrogen 2.86%, and nitrogen 66.67% of the total mass.
- 🌡 The gases are at standard temperature, which is 0 degrees Celsius or 273 Kelvin.
- 🧪 The task is to calculate the total pressure in the container and the partial pressures of each gas.
- 📚 The concept of partial pressure is introduced, which is the pressure exerted by an individual gas in a mixture.
- 🔍 The process begins by determining the total moles of gas present in the container.
- ⚖️ The molar mass of each gas (O2, H2, N2) is used to calculate the moles of each gas from their mass percentages.
- 🔢 It is found that there are 50 moles of N2, 20 moles of O2, and 30 moles of H2, summing up to 100 moles of gas in total.
- 📐 The ideal gas law PV = nRT is applied to calculate the total pressure, where n is the number of moles, R is the gas constant, T is the temperature in Kelvin, and P is the pressure.
- 📉 The calculated total pressure is 56,746 pascals, which is equivalent to 0.56 atmospheres.
- 🔄 The partial pressures are determined by the proportion of moles each gas contributes to the total, resulting in 0.112 atmospheres for oxygen, 0.28 atmospheres for nitrogen, and 0.168 atmospheres for hydrogen.
- 🔍 The final takeaway is that each gas contributes to the total pressure according to its mole fraction, regardless of its mass fraction.
Q & A
What is the total mass of gas in the container?
-The total mass of gas in the container is 2.1 kilograms.
What are the percentages of oxygen, hydrogen, and nitrogen in the container by mass?
-The percentages of oxygen, hydrogen, and nitrogen in the container by mass are 30.48%, 2.86%, and 66.67% respectively.
How many moles of nitrogen are in the container?
-There are 50 moles of nitrogen (N2) in the container.
What is the molar mass of a nitrogen molecule (N2)?
-The molar mass of a nitrogen molecule (N2) is 28 grams per mole.
How many moles of oxygen are in the container?
-There are 20 moles of oxygen (O2) in the container.
What is the molar mass of an oxygen molecule (O2)?
-The molar mass of an oxygen molecule (O2) is 32 grams per mole.
How many moles of hydrogen are in the container?
-There are 30 moles of hydrogen (H2) in the container.
What is the molar mass of a hydrogen molecule (H2)?
-The molar mass of a hydrogen molecule (H2) is 2 grams per mole.
What is the total pressure in the container in pascals?
-The total pressure in the container is 56,746 pascals.
How many atmospheres is the total pressure in the container?
-The total pressure in the container is equivalent to 0.56 atmospheres.
What is the partial pressure of oxygen in the container?
-The partial pressure of oxygen in the container is 0.112 atmospheres.
What is the partial pressure of nitrogen in the container?
-The partial pressure of nitrogen in the container is approximately 0.28 atmospheres.
What is the partial pressure of hydrogen in the container?
-The partial pressure of hydrogen in the container is 0.168 atmospheres.
How does the total pressure relate to the partial pressures of the individual gases?
-The total pressure in the container is the sum of the partial pressures of oxygen, hydrogen, and nitrogen.
Outlines
🟠 Understanding the Problem: Gas Mixture in a Container
We are given a scenario involving a 4-cubic-meter container (like a balloon) with a mixture of oxygen, hydrogen, and nitrogen gases. The total gas mass is 2.1 kilograms, with 30.48% oxygen, 2.86% hydrogen, and 66.67% nitrogen. The goal is to determine the total pressure and partial pressures of each gas at standard temperature (0°C or 273 Kelvin). The process involves calculating the total number of moles of each gas type by using their respective masses and molar masses, then using the ideal gas law (PV=nRT) to find the pressures.
🔵 Calculating Moles of Each Gas
To find the total number of moles of each gas: 66.67% of the total mass is nitrogen (1400 grams), which has a molar mass of 28 grams/mole, resulting in 50 moles of nitrogen. For oxygen, 30.48% of the total mass is 640 grams, with a molar mass of 32 grams/mole, giving 20 moles of oxygen. Lastly, 2.86% of the total mass is hydrogen (60 grams), with a molar mass of 2 grams/mole, resulting in 30 moles of hydrogen. Summing these, we get 100 moles of gas in the container.
🟢 Total Pressure Calculation Using Ideal Gas Law
Using the ideal gas law (PV=nRT) and the values calculated: total moles (100 moles), volume (4 cubic meters), temperature (273 Kelvin), and gas constant (8.3145 meters cubed pascals per mole Kelvin), we solve for the total pressure (P). This gives P = 56,746 pascals (56.746 kilopascals or 0.56 atmospheres). Dimensional analysis confirms the correctness of units, resulting in pressure units in pascals.
🟡 Determining Partial Pressures
Partial pressures are found by multiplying the total pressure by the mole fraction of each gas. For oxygen (20% of total moles), the partial pressure is 0.112 atmospheres (20% of 0.56). For nitrogen (50% of total moles), the partial pressure is 0.28 atmospheres. For hydrogen (30% of total moles), the partial pressure is 0.168 atmospheres. Summing these partial pressures confirms the total pressure of 0.56 atmospheres, demonstrating that each gas's contribution is proportional to its mole fraction.
Mindmap
Keywords
💡Container
💡Molecules of Gas
💡Total Pressure
💡Partial Pressure
💡Moles
💡Molar Mass
💡Ideal Gas Law
💡Temperature
💡Gas Constant
💡Percentage Composition
Highlights
A four-meter cubed container filled with a mixture of oxygen, hydrogen, and nitrogen gases is considered.
The total mass of the gas mixture is 2.1 kilograms, with specific percentages for each gas type.
The calculation of moles of nitrogen is demonstrated using its percentage in the mixture and molar mass.
50 moles of nitrogen (N2) are determined, highlighting the relationship between mass and moles.
The process for calculating moles of oxygen involves using its percentage of the total mass and molar mass.
20 moles of oxygen (O2) are calculated, emphasizing the atomic mass of oxygen in the calculation.
Hydrogen's moles are determined by its percentage in the mixture and its atomic mass, resulting in 30 moles of hydrogen (H2).
The concept of partial pressure and its relation to the number of gas particles is introduced.
The total number of moles of gas particles in the container is summed up to be 100 moles.
The application of the ideal gas law (PV=nRT) to calculate the total pressure in the container is explained.
The total pressure is calculated to be 56,746 pascals, demonstrating the use of R and temperature in the formula.
Conversion of the total pressure to atmospheres and kilopascals for different unit perspectives.
The method to find the partial pressure of each gas based on their moles percentage in the total mixture is detailed.
The partial pressures of oxygen, nitrogen, and hydrogen are calculated, showing their individual contributions to the total pressure.
The importance of understanding that each molecule contributes to the total pressure proportional to its moles is emphasized.
A verification that the sum of partial pressures equals the total pressure, reinforcing the concept of ideal gas behavior.
The conclusion highlights the significance of mole count over mass in determining partial pressures in an ideal gas mixture.
Transcripts
Browse More Related Video

Introduction to partial pressure | Gases and kinetic molecular theory | Chemistry | Khan Academy
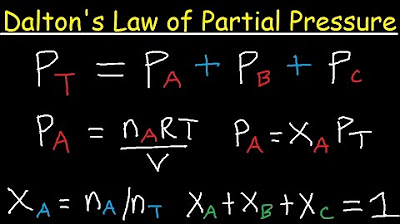
Dalton's Law of Partial Pressure Problems & Examples - Chemistry

9.3 Additional Gas Laws | Dalton's Law and Graham's Law | High School Chemistry

Daltons Law | Partial Pressures

Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion
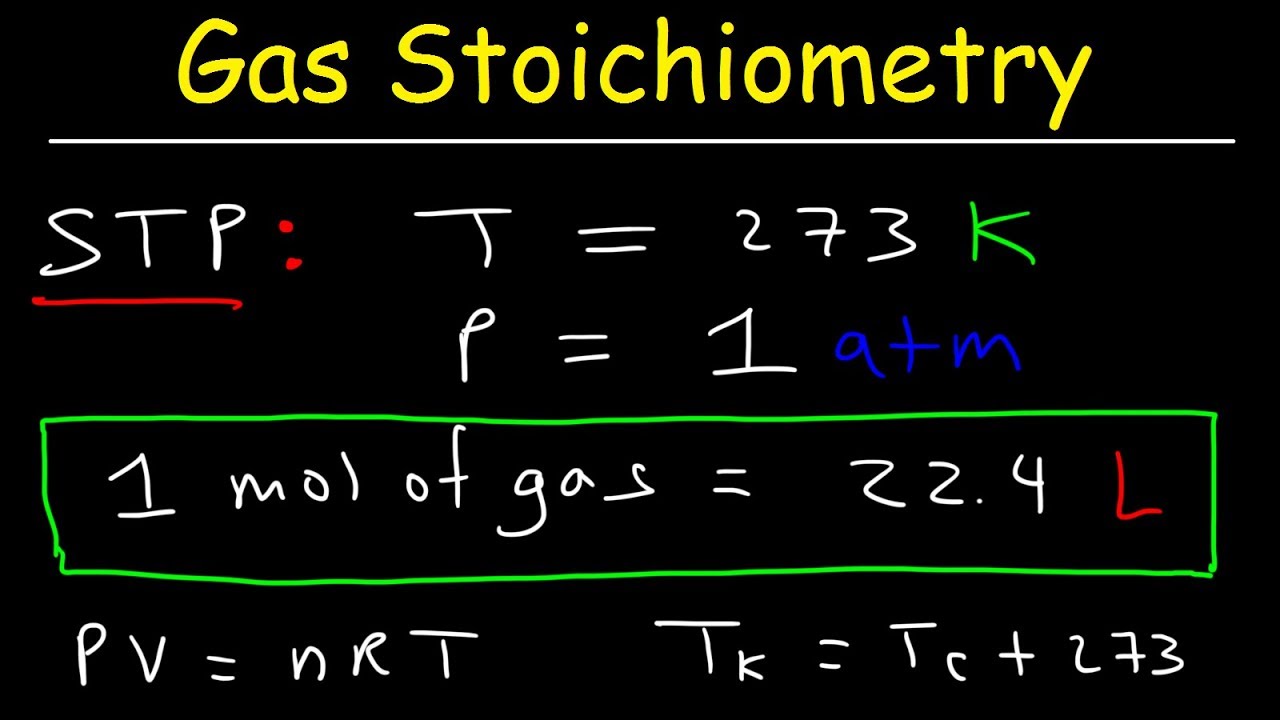
Gas Stoichiometry Problems
5.0 / 5 (0 votes)
Thanks for rating: