Introduction to partial pressure | Gases and kinetic molecular theory | Chemistry | Khan Academy
TLDRThis video explains the concept of partial pressure in gas mixtures, focusing on ideal gases. It illustrates how gas molecules behave independently and exert pressure on their container. By adding oxygen gas to a container with nitrogen gas, the total pressure increases. Using Dalton's Law of Partial Pressures, the video demonstrates how to calculate individual gas pressures within a mixture. The example shows that the total pressure is the sum of the partial pressures, and the pressure of each gas can be determined using the ideal gas law, given the number of moles, temperature, and volume remain constant.
Takeaways
- 🌪️ Partial pressure is the pressure exerted by a single gas in a mixture of gases, assuming ideal gas behavior.
- 🚀 Ideal gases are characterized by their molecules behaving independently, without interacting with each other.
- 🎨 The illustration of gas molecules in a container shows nitrogen as purple and oxygen as pink, with their velocities indicated by arrows.
- 🔍 The pressure in the container increases with the addition of another gas, such as oxygen, without changing the volume or temperature.
- 📈 The total pressure of a gas mixture is the sum of the partial pressures of each individual gas present, as described by Dalton's Law of Partial Pressures.
- ⚖️ Dalton's Law states that the total pressure is equal to the sum of the partial pressures of all gases in the mixture.
- 🔄 The partial pressure of a gas remains constant if the number of moles, temperature, or volume remains unchanged, even when other gases are added.
- 📚 The ideal gas law, PV = nRT, can be rewritten in terms of pressure as P = nRT/V, showing how pressure is related to the number of moles, temperature, and volume.
- 🧩 By knowing the total pressure and the partial pressures of other gases, one can calculate the unknown partial pressure of a specific gas in the mixture.
- 📉 In the example, the partial pressure of nitrogen remains at 2.0 atmospheres even after adding oxygen, demonstrating the independence of gas behavior.
- 📝 The partial pressure of oxygen is found by subtracting the known partial pressure of nitrogen from the total pressure, resulting in 0.5 atmospheres.
Q & A
What is partial pressure?
-Partial pressure is the pressure exerted by a single gas within a mixture of gases when they behave like ideal gases.
What is the characteristic behavior of ideal gas molecules?
-Ideal gas molecules behave independently, meaning they do not interact with each other and move in random directions with varying velocities.
How does the addition of another gas to a container affect the pressure?
-Adding another gas to a container increases the total number of gas molecules, which in turn increases the total pressure within the container.
What is Dalton's Law of partial pressures?
-Dalton's Law of partial pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of the individual gases in the mixture.
How can you calculate the partial pressure of a gas in a mixture?
-You can calculate the partial pressure of a gas in a mixture by subtracting the known partial pressures of other gases from the total pressure.
Why does the partial pressure of nitrogen remain the same when oxygen is added to the container?
-The partial pressure of nitrogen remains the same because the number of moles, temperature, and volume of the nitrogen gas have not changed, according to the ideal gas law.
What is the ideal gas law and how is it related to partial pressure?
-The ideal gas law is PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature. It is related to partial pressure as it shows how the pressure exerted by a gas is dependent on the number of moles, volume, and temperature.
How does temperature affect the partial pressure of a gas?
-According to the ideal gas law, if the volume and the number of moles of a gas remain constant, an increase in temperature will result in an increase in pressure, and vice versa.
Can the partial pressure of a gas be zero?
-The partial pressure of a gas cannot be zero as long as there are gas molecules present in the container, as they will always exert some pressure by colliding with the walls of the container.
What happens to the total pressure if the volume of the container is decreased while keeping the number of moles and temperature constant?
-If the volume of the container is decreased while keeping the number of moles and temperature constant, the total pressure will increase according to Boyle's Law, which is a specific case of the ideal gas law.
How can you determine the unknown partial pressure of a gas in a mixture if you know the total pressure and the partial pressures of other gases?
-You can determine the unknown partial pressure by using Dalton's Law and subtracting the sum of the known partial pressures from the total pressure.
Outlines
🌪️ Partial Pressure in Gas Mixtures
This paragraph explains the concept of partial pressure, which is the pressure exerted by a single gas in a mixture of gases, assuming ideal gas behavior where molecules act independently. The example of a container with nitrogen gas (N2) is used to illustrate how gas molecules move and create pressure by colliding with the container walls. The introduction of oxygen gas (O2) into the container increases the total pressure without affecting the partial pressure of the nitrogen, demonstrating the additive nature of partial pressures according to Dalton's Law of Partial Pressures. The total pressure is calculated as the sum of the partial pressures of all gases present.
🔍 Calculating Partial Pressure Using Dalton's Law
Building on the previous explanation, this paragraph delves into how to calculate the partial pressure of a specific gas in a mixture using Dalton's Law. It emphasizes that the partial pressure of nitrogen remains constant at 2.0 atmospheres even after adding oxygen, due to the ideal gas law (PV=nRT), which states that pressure changes only with changes in the number of moles, temperature, or volume. The paragraph concludes by showing how to rearrange the equation to solve for the partial pressure of oxygen, which is found to be 0.5 atmospheres, thus providing a clear method for determining unknown partial pressures when the total pressure and other partial pressures are known.
Mindmap
Keywords
💡Partial pressure
💡Ideal gas
💡Dalton's Law of Partial Pressures
💡Total pressure
💡Nitrogen gas (N2)
💡Oxygen gas (O2)
💡Pressure
💡Volume
💡Temperature
💡Ideal Gas Law (PV=nRT)
Highlights
Partial pressure is the pressure exerted by one gas in a mixture of gases.
The concept is applicable to ideal gases, where gas molecules behave independently.
Gas molecules in a mixture do not interact with each other and move randomly.
An illustration of gas molecules whizzing around in a container is provided.
The pressure from gas molecules bouncing off container walls is explained.
The example of nitrogen gas (N2) in a container with a pressure of two atmospheres is given.
Adding oxygen gas to the container increases the total pressure to 2.5 atmospheres.
The total pressure is the sum of the partial pressures of all gases in the mixture.
Dalton's Law of partial pressures is introduced as a method to calculate individual gas pressures.
The equation for partial pressure is presented as the sum of all partial pressures equaling the total pressure.
The partial pressure of nitrogen remains 2.0 atmospheres even after adding oxygen, due to ideal gas behavior.
The ideal gas law PV=nRT is mentioned, relating pressure to moles, temperature, and volume.
The partial pressure of nitrogen is unaffected by adding another gas, as long as moles, temperature, and volume remain constant.
The partial pressure of oxygen is calculated by subtracting the nitrogen partial pressure from the total pressure.
The partial pressure of oxygen is determined to be 0.5 atmospheres.
Dalton's Law is used to find the partial pressure of one gas when the total pressure and other partial pressures are known.
Transcripts
Browse More Related Video

Partial pressure example | Chemistry | Khan Academy

9.3 Additional Gas Laws | Dalton's Law and Graham's Law | High School Chemistry
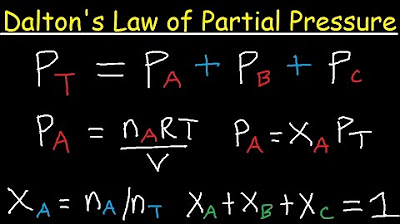
Dalton's Law of Partial Pressure Problems & Examples - Chemistry

Daltons Law | Partial Pressures
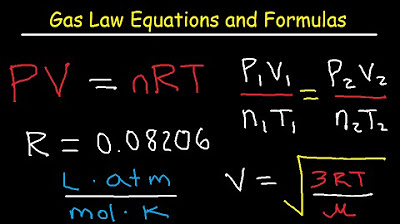
Gas Laws - Equations and Formulas
Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion
5.0 / 5 (0 votes)
Thanks for rating: