Henry's Law | Henry's Law Constant | Henry's Law Numericals
TLDRThis educational script introduces Henry's Law, a fundamental concept in chemistry relating the solubility of a gas in a liquid to the pressure of the gas above the liquid. It begins with basic concepts like friction and mole fraction, using relatable examples such as dividing a cake to explain these ideas. The script then explains concentration and pressure, leading to the definition of Henry's Law, which states that gas solubility is directly proportional to the pressure above the liquid surface. It also discusses the Henry constant (KH), which varies with the nature of the gas and solvent, and provides a step-by-step solution to a competitive exam problem involving the solubility of nitrogen gas in water, illustrating the application of Henry's Law.
Takeaways
- 🍰 The script begins with an analogy using a cake to explain the concept of mole fraction, where the 'friction' of Mr. Najim and Mr. Ali eating different parts of the cake represents their respective mole fractions.
- 🧪 It then introduces the concept of mole fraction in a solution, using the example of a solution containing P, Q, and R with different moles to illustrate how to calculate the mole fraction of each component.
- 📚 The script explains the concept of concentration, emphasizing that it measures how many moles of solute are dissolved in a given amount of solvent, using the example of sugar dissolved in water.
- 🔨 The importance of pressure is discussed, particularly how it affects the solubility of gases, with the example of gas particles colliding with the walls of a container to exert pressure.
- ⚖️ Henry's Law is introduced, stating that the solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid surface.
- 🔍 The script clarifies that the solubility of gas particles increases with an increase in pressure due to more collisions per unit area, leading to easier dissolution.
- 📘 Mathematically, Henry's Law is expressed as the pressure being directly proportional to the mole fraction of the gas (X_gas), with the proportionality constant known as the Henry's constant (KH).
- 🔑 The Henry's constant (KH) is highlighted as being dependent on the nature of the gas and the solvent, and its unit is the same as that of pressure, such as ATM.
- 📉 The script points out that the mole fraction is inversely proportional to the Henry's constant, meaning a higher KH results in lower solubility.
- 🌡️ An example problem from a competitive exam is solved, demonstrating the application of Henry's Law to calculate the solubility of nitrogen gas in water at a given temperature and pressure.
- 📝 The final takeaway emphasizes the importance of understanding the mole fraction of nitrogen in air and water, as well as the partial pressure of nitrogen, to solve problems related to gas solubility.
Q & A
What is the basic concept of Henry's law?
-Henry's law states that the solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid surface at constant temperature.
How is 'friction' in the context of the cake example related to mole fraction?
-In the cake example, 'friction' is a metaphor for mole fraction. If Mr. Najim eats one piece out of four, his 'friction' is one-fourth, analogous to a mole fraction of 1/4.
What is the mole fraction of a component in a solution?
-The mole fraction of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all components in the solution.
How is concentration different from mole fraction?
-Concentration measures the amount of solute dissolved in a given amount of solvent, whereas mole fraction is a dimensionless quantity that represents the proportion of a particular component in a mixture.
What is the relationship between pressure and the solubility of gas particles according to Henry's law?
-According to Henry's law, the solubility of gas particles is directly proportional to the pressure of the gas above the liquid surface.
What is the mathematical representation of Henry's law?
-The mathematical representation of Henry's law is P = kH * X, where P is the pressure, kH is the Henry's constant, and X is the mole fraction of the gas in the solution.
What is the Henry constant (kH) and how does it vary?
-The Henry constant (kH) is a proportionality constant in Henry's law that relates the mole fraction of a gas in a solution to its partial pressure. It varies depending on the nature of the gas and the solvent.
How does the mole fraction of a gas in a solution relate to the Henry constant?
-The mole fraction of a gas in a solution is inversely proportional to the Henry constant, meaning that a higher kH results in a lower solubility of the gas in the solution.
What is the significance of the mole fraction of nitrogen gas in air being 0.8 in the context of the IIT exam problem?
-The mole fraction of nitrogen gas in air being 0.8 is used to calculate the partial pressure of nitrogen gas exerted on the surface of water, which is essential for determining the solubility of nitrogen gas in water.
How many moles of nitrogen gas dissolve in 10 moles of water at 298 Kelvin and 5 ATM according to the IIT exam problem?
-According to the calculations in the IIT exam problem, 4 * 10^-4 moles of nitrogen gas dissolve in 10 moles of water at 298 Kelvin and 5 ATM.
Outlines
📚 Introduction to Basic Concepts for Understanding Henry's Law
This paragraph introduces Henry's Law by explaining basic concepts such as friction and mole fraction. The analogy of dividing a cake into pieces is used to illustrate how mole fraction works, followed by an example involving a solution with different moles of substances P, Q, and R. The concept of concentration is explained with an example of sugar solutions, and the relationship between pressure and gas particle collisions is also discussed.
🔍 Understanding Henry's Law and Solubility
This paragraph focuses on Henry's Law, explaining how the solubility of gas particles is directly proportional to the pressure above the liquid surface. The explanation includes a comparison of gas solubility at high and low pressures, reinforcing the concept that increased pressure leads to increased solubility due to more frequent particle collisions. The mathematical representation of Henry's Law is introduced, along with the concept of the Henry constant (KH).
🧪 Solving a Numerical Problem Using Henry's Law
This paragraph presents a numerical problem related to Henry's Law, involving the solubility of nitrogen gas in water. The given data includes the Henry constant, mole fraction of nitrogen gas in air, and total pressure. The process involves calculating the partial pressure of nitrogen gas and using Henry's Law to find the mole fraction in water. The final step involves determining the number of moles of nitrogen gas dissolved in water, illustrating the practical application of the concepts discussed.
Mindmap
Keywords
💡Henry's Law
💡Friction
💡Mole Fraction
💡Concentration
💡Pressure
💡Gas Solubility
💡Henry Constant (KH)
💡Partial Pressure
💡Mole Fraction in Air
💡Numerical Problem
Highlights
Introduction to Henry's Law and basic concepts like friction, mole fraction, concentration, and pressure.
Friction concept illustrated with a cake divided into four parts, eaten by two people, to explain mole fraction.
Explanation of mole fraction using a solution with moles of P, Q, and R to find the mole fraction of T.
Concentration defined as the measure of solute dissolved in a given amount of solvent.
Pressure defined as force per unit area, with examples of gas particles colliding with cylinder walls.
Henry's Law stated as the solubility of a gas in a liquid being directly proportional to the pressure above the liquid surface.
Henry's Law mathematically expressed as pressure proportional to mole fraction of the gas (X_gas) with Henry's constant (KH).
Henry's constant (KH) depends on the nature of the gas and solvent, with examples given for hydrogen gas and carbon dioxide.
Mole fraction (X) is unitless, and thus the unit of KH matches the pressure unit, such as ATM.
Henry's Law can be rewritten to show mole fraction inversely proportional to the Henry's constant.
Example problem solving to determine which gas is more soluble in water at 298 Kelvin.
Explanation of a numerical problem from the IIT competitive exam involving the solubility of nitrogen gas in water.
Use of Dalton's Law to find the partial pressure of nitrogen gas exerted on the surface of water.
Calculation of mole fraction of nitrogen gas in water using the given Henry's constant and partial pressure.
Determination of the number of moles of nitrogen gas dissolved in water, considering the mole fraction and total moles of water.
Conclusion that 4x10^-4 moles of nitrogen gas dissolve in water under the given conditions.
Transcripts
Browse More Related Video

Henry's Law Explained - Gas Solubility & Partial Pressure - Chemistry Problems

Pressure and Gas Solubility (Henry's Law)

Daltons Law | Partial Pressures

Phase diagrams | States of matter and intermolecular forces | Chemistry | Khan Academy

Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion
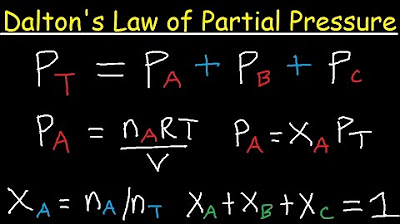
Dalton's Law of Partial Pressure Problems & Examples - Chemistry
5.0 / 5 (0 votes)
Thanks for rating: