2.3 Precision and Accuracy | High School Chemistry
TLDRThis high school chemistry lesson distinguishes between precision and accuracy in measurement. Precision refers to the repeatability of measurements, ensuring they are close to each other, while accuracy relates to how close these measurements are to the true value. Examples using a target analogy illustrate the concepts, emphasizing the importance of both in scientific and engineering contexts.
Takeaways
- 🔍 The lesson discusses the concepts of precision and accuracy in the context of measurement, which are crucial in high school chemistry.
- 📚 The script is part of a larger high school chemistry playlist, with new content released weekly for the 2020-2021 school year.
- 🔔 Viewers are encouraged to subscribe and enable notifications to stay updated with the playlist.
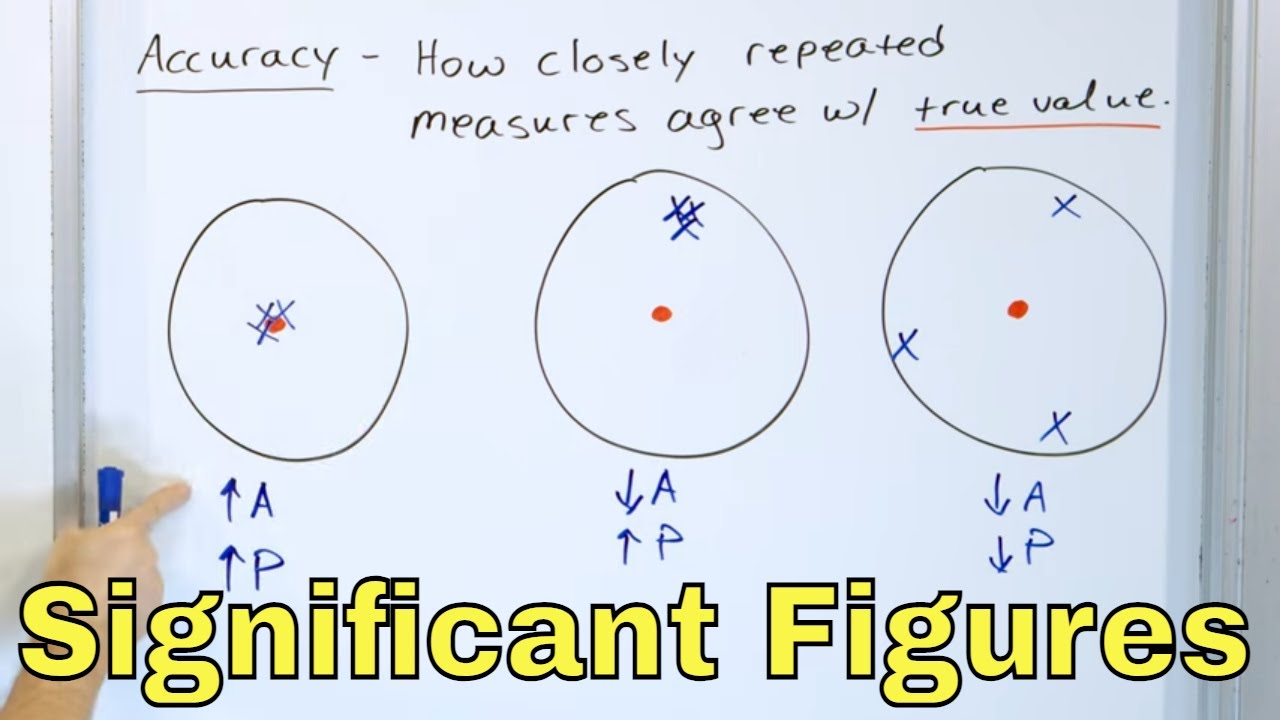
- 🔬 Precision refers to the repeatability of measurements, indicating how closely multiple measurements are to each other, regardless of their correctness.
- 🎯 Accuracy, on the other hand, is about how close a measurement is to the true value.
- 💯 An example of both precision and accuracy is hitting a bullseye with multiple arrows, indicating correct and closely grouped shots.
- 🎯 An example of precision without accuracy is a group of arrows that are closely grouped but miss the bullseye entirely.
- ❌ An example of neither precision nor accuracy is a set of arrows that neither hit the bullseye nor are grouped closely together.
- 📊 The script uses the analogy of target shooting to illustrate the differences between precision and accuracy.
- 📈 The script explains that a large set of data can be accurate if its average is close to the true value, but it may not be precise if the data points are widely spread.
- 📘 The lesson concludes with a reminder that understanding precision and accuracy is important for anyone studying chemistry or related sciences.
Q & A
What is the main topic of this third lesson in the high school chemistry playlist?
-The main topic of this third lesson is precision and accuracy in measurement.
Why is it important to distinguish between precision and accuracy in scientific and engineering contexts?
-In scientific and engineering contexts, precision and accuracy are not synonymous terms. Precision refers to the repeatability of measurements, indicating how close multiple measurements are to each other, while accuracy refers to how close the measurements are to the true value.
What does precision measure in the context of measurements?
-Precision measures the repeatability of a measurement, indicating how close a set of measurements are to each other.
How is accuracy defined in the context of measurements?
-Accuracy is defined as how close the measurements are to the true value.
What is an example given in the script to illustrate the difference between precision and accuracy?
-The script uses the example of shooting arrows at a bullseye. If all arrows hit the bullseye, they are both accurate and precise. If they are close together but miss the bullseye, they are precise but not accurate. If they neither hit the bullseye nor are close together, they are neither precise nor accurate.
What is the significance of a set of measurements being both precise and accurate?
-A set of measurements being both precise and accurate indicates that the measurements are not only consistent with each other but also close to the true value, which is ideal in scientific and engineering contexts.
How can you determine if a set of measurements is accurate but not precise?
-A set of measurements can be determined as accurate but not precise if the average of the measurements corresponds to the true value, but the individual measurements are spread out widely.
What might happen if a large set of data has a large spread but the average corresponds to the true value?
-In such a case, the data set would be considered accurate because the average matches the true value, but it would not be considered precise due to the large spread of the individual measurements.
What does the script suggest about the relationship between the spread of data and precision?
-The script suggests that if the spread of data is large, even if the average corresponds to the true value, the data set would not be considered precise.
What advice does the script give for those interested in further learning about precision and accuracy?
-The script advises those interested in further learning to check out the premium course on chatsprep.com for practice materials associated with the topic.
Outlines
📏 Introduction to Precision and Accuracy in Measurement
This paragraph introduces the third lesson in a series on measurement within a high school chemistry playlist. The focus is on the concepts of precision and accuracy, which are often mistakenly used interchangeably in everyday language but have distinct meanings in scientific and engineering contexts. Precision refers to the repeatability of measurements, indicating how close multiple measurements are to each other, while not necessarily being the correct or true value. Accuracy, in contrast, pertains to how close a measurement is to the true value. The paragraph emphasizes the importance of understanding the difference between these two concepts.
🎯 Understanding Precision Through Examples
This section delves deeper into the concept of precision with the help of a target analogy. It explains that if multiple arrows hit close to each other, they represent a precise set of measurements, even if they do not hit the bullseye, which would indicate accuracy. The paragraph contrasts this with a scenario where arrows are grouped closely but miss the target entirely, illustrating a case of high precision but low accuracy.
📐 The Relationship Between Precision, Accuracy, and True Value
This paragraph further explores the relationship between precision, accuracy, and the true value of a measurement. It uses the example of measuring height to illustrate accuracy, where a measurement close to the actual height is considered accurate. The paragraph also discusses the scenario where a large set of data might have an average that corresponds to the true value, indicating accuracy, but if the individual measurements are spread out widely, the set is not considered precise.
Mindmap
Keywords
💡Precision
💡Accuracy
💡Measurement
💡Significant Figures
💡Scientific Notation
💡Units and Conversions
💡True Value
💡Repeatability
💡High School Chemistry
💡Bullseye
💡Spread
Highlights
The third lesson in the chemistry playlist focuses on precision and accuracy in measurement.
Precision and accuracy are often used interchangeably in everyday language, but they are not synonymous in science and engineering.
Precision refers to the repeatability of a measurement, indicating how close multiple measurements are to each other.
Accuracy is about being close to the true value, which is the correct measurement corresponding to reality.
An example of both precision and accuracy is hitting the bullseye with all shots, indicating they are both close together and to the target.
A group of shots close together but missing the bullseye demonstrates precision without accuracy.
Shots that neither hit the bullseye nor are close to each other lack both precision and accuracy.
The concept of precision and accuracy can be illustrated with target practice, where the grouping of shots shows their relationship.
Taking the average of a large set of data can indicate accuracy if it corresponds to the true value.
The spread of measurements is crucial; a large spread can affect the precision even if the average is accurate.
The video is part of a high school chemistry playlist released weekly for the 2020-2021 school year.
Viewers are encouraged to subscribe to the channel and click the bell notification for updates on new lessons.
The lesson differentiates between precision as the closeness of measurements to each other and accuracy as their closeness to the true value.
An accurate measurement is one that is close to the true value, like measuring someone's height correctly.
The importance of understanding the difference between precision and accuracy in scientific measurements is emphasized.
The lesson uses the analogy of target shooting to visually demonstrate the concepts of precision and accuracy.
The video provides a clear explanation of how precision and accuracy are distinct but related in the context of measurement.
For further study materials, viewers are directed to check out the premium course on chatsprep.com.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: