Balancing Chemical Equations With Fractions | How to Pass Chemistry
TLDRThe video script offers a step-by-step guide on balancing chemical equations involving fractions, particularly in combustion reactions. It emphasizes the importance of identifying the number of atoms for each element in reactants and products. The process involves initially balancing carbon, then hydrogen, and finally addressing the odd number of oxygen atoms by introducing fractions. The script concludes with a method to eliminate the fraction by multiplying all coefficients by 2, resulting in a balanced equation.
Takeaways
- 🔍 The script discusses the process of balancing a chemical equation when fractions are involved.
- 📝 The first step is to identify the number of each element in both reactants and products.
- 🌐 For the reactants, there are 6 carbons, 14 hydrogens, and 2 oxygens.
- 🚫 On the product side, initially, there is 1 carbon, 2 hydrogens, and 3 oxygens.
- 🔄 When balancing, carbon should be addressed first, followed by hydrogen, and oxygen last.
- ⚖️ A coefficient of 6 is placed in front of CO2 to balance the carbon atoms.
- 🔄 After balancing carbon, the script moves to balance hydrogen, using a coefficient of 7 for H2O.
- 🧩 After adjusting for hydrogen, the oxygen count on the product side becomes 19, which is an odd number.
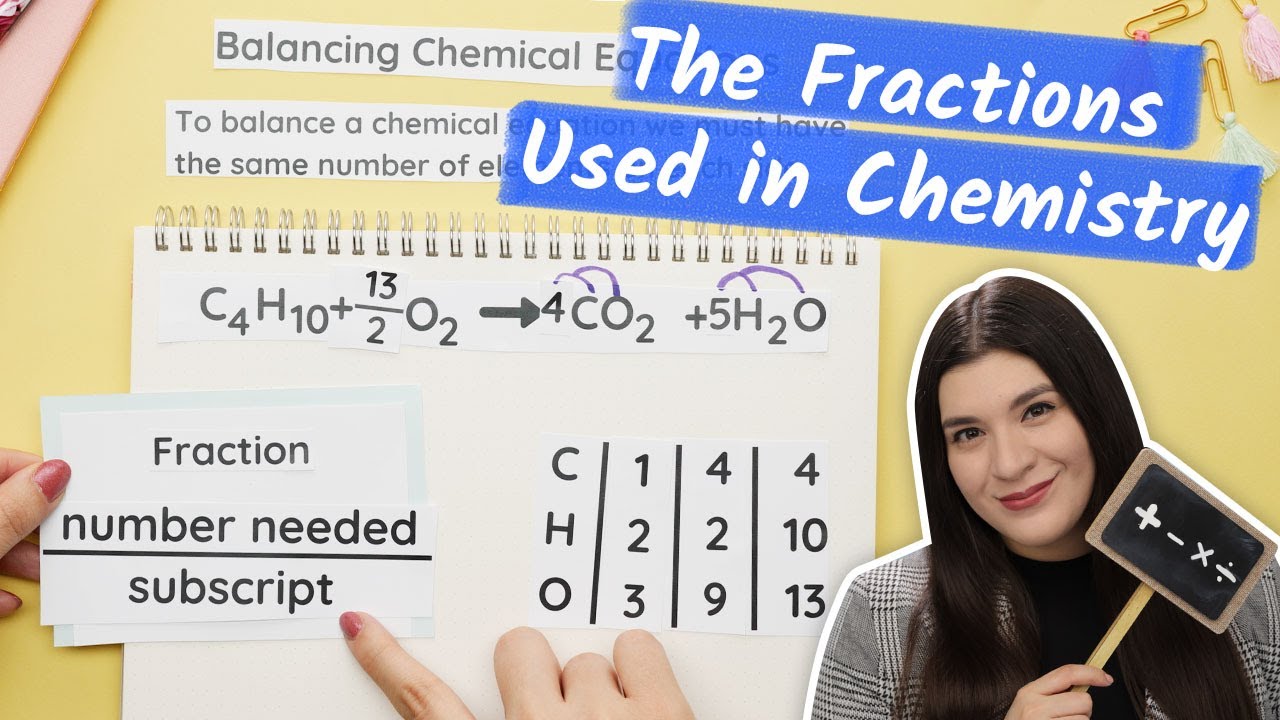
- 📉 To handle the odd number of oxygen atoms, the script introduces a fraction, specifically halves.
- 🔗 The fraction is used to eliminate the odd number by placing 19 halves in front of the oxygen-containing products.
- 🔄 The final step is to multiply all coefficients by 2 to eliminate the fraction and achieve a whole number balance.
- 📋 The balanced equation is achieved by distributing the multiplication across all reactants and products, resulting in whole numbers.
Q & A
Why do some combustion reactions involve fractions in their balanced equations?
-Some combustion reactions involve fractions because the number of atoms on the reactant side may not be a multiple of the number of atoms on the product side, leading to an odd number of atoms that cannot be balanced without using fractions.
What is the first step when dealing with an unbalanced chemical equation that includes fractions?
-The first step is to identify the number of each element present in the reactants and products, which helps in understanding the imbalance and planning the balancing process.
Why is it recommended to leave oxygen for the very end when balancing chemical equations?
-Oxygen is often left for the end because it frequently appears in many compounds and can complicate the balancing process if addressed too early. It's a strategic approach to simplify the balancing steps.
Why is hydrogen usually left for the second to last in the balancing process?
-Hydrogen is left for the second to last because it is commonly found in many organic compounds and its balancing can be more straightforward once other elements are accounted for, making the process more manageable.
How does one begin to balance carbon in the given script?
-To balance carbon, you identify the number of carbon atoms on both sides of the equation and place a coefficient in front of the compound containing carbon on the product side to match the number of carbon atoms.
What is the significance of keeping the compound together when balancing chemical equations?
-Keeping the compound together is important because it maintains the integrity of the chemical species and ensures that the law of conservation of mass is respected, meaning the types and numbers of atoms remain constant.
How do you determine the coefficient for hydrogen when balancing the equation?
-You determine the coefficient for hydrogen by finding a number that, when multiplied by the number of hydrogen atoms in the compound, gives you the total number of hydrogen atoms on the reactant side.
What is the role of fractions in balancing chemical equations?
-Fractions are used when the number of atoms of a particular element cannot be evenly distributed on both sides of the equation, resulting in an odd number that requires a fractional coefficient to balance the equation.
How does the process of multiplying all subscripts by 2 help in eliminating fractions in a balanced equation?
-Multiplying all subscripts by 2 is a method to clear the fraction by making the denominator a whole number, thus simplifying the equation to have only whole number coefficients.
What is the final step in the script for balancing the chemical equation using fractions?
-The final step is to multiply all the coefficients by 2 to eliminate the fraction, ensuring that all elements are balanced with whole numbers, resulting in a fully balanced chemical equation.
Why is it important to recount the number of oxygen atoms after each balancing step?
-Recounting the number of oxygen atoms after each step is crucial to ensure that the balance is maintained across all elements and to identify any discrepancies that may require further adjustment.
Outlines
🔍 Balancing Chemical Equations with Fractions
This paragraph explains the process of balancing a chemical equation that involves fractions. It begins by emphasizing the importance of identifying the number of each element in both reactants and products. The example provided demonstrates how to balance carbon first, followed by hydrogen, and finally addressing the oxygen with a fraction. The paragraph details the step-by-step approach, including the placement of coefficients in front of compounds to maintain their integrity and the use of fractions when an odd number of atoms cannot be evenly distributed. The final step involves eliminating the fraction by multiplying all subscripts by two, resulting in a balanced equation.
Mindmap
Keywords
💡Combustion Reactions
💡Fractions
💡Unbalanced Equation
💡Element
💡Reactants
💡Products
💡Balancing Equations
💡Carbon Dioxide (CO2)
💡Hydrogen (H)
💡Oxygen (O)
💡Stoichiometry
Highlights
Introduction to handling fractions in combustion reaction equations.
Explanation of the importance of identifying the number of elements in reactants and products.
Specific identification of carbon, hydrogen, and oxygen in the given example.
Strategy to prioritize balancing elements, starting with carbon and ending with oxygen.
Demonstration of balancing carbon by adjusting the coefficient of CO2.
Clarification on maintaining the integrity of compounds when adjusting coefficients.
Recalibration of oxygen count after adjusting for carbon balance.
Approach to balancing hydrogen by finding a multiplier for the given coefficient.
Use of multiplication to adjust coefficients and achieve hydrogen balance.
Recognition of the inevitable odd number of oxygen atoms after balancing other elements.
Introduction of the concept of using fractions to balance equations with an odd number of atoms.
Method to place a fraction to balance the odd number of oxygen atoms.
Technique to eliminate the fraction by multiplying all coefficients by the denominator.
Final step of simplifying the equation to achieve a fully balanced chemical equation.
Emphasis on the importance of distributing the multiplier across all parts of the compound.
Result of the balanced equation using fractions, showcasing the final coefficients.
Transcripts
Browse More Related Video
Introduction to Balancing Chemical Equations

How To Balance Combustion Reactions
Balancing Combustion Reactions

Balancing another combustion reaction | Chemical reactions | High school chemistry | Khan Academy

01 - Introduction to the Algebraic Method for Balancing Chemical Equations
How to Use Fractions in Chemistry
5.0 / 5 (0 votes)
Thanks for rating: