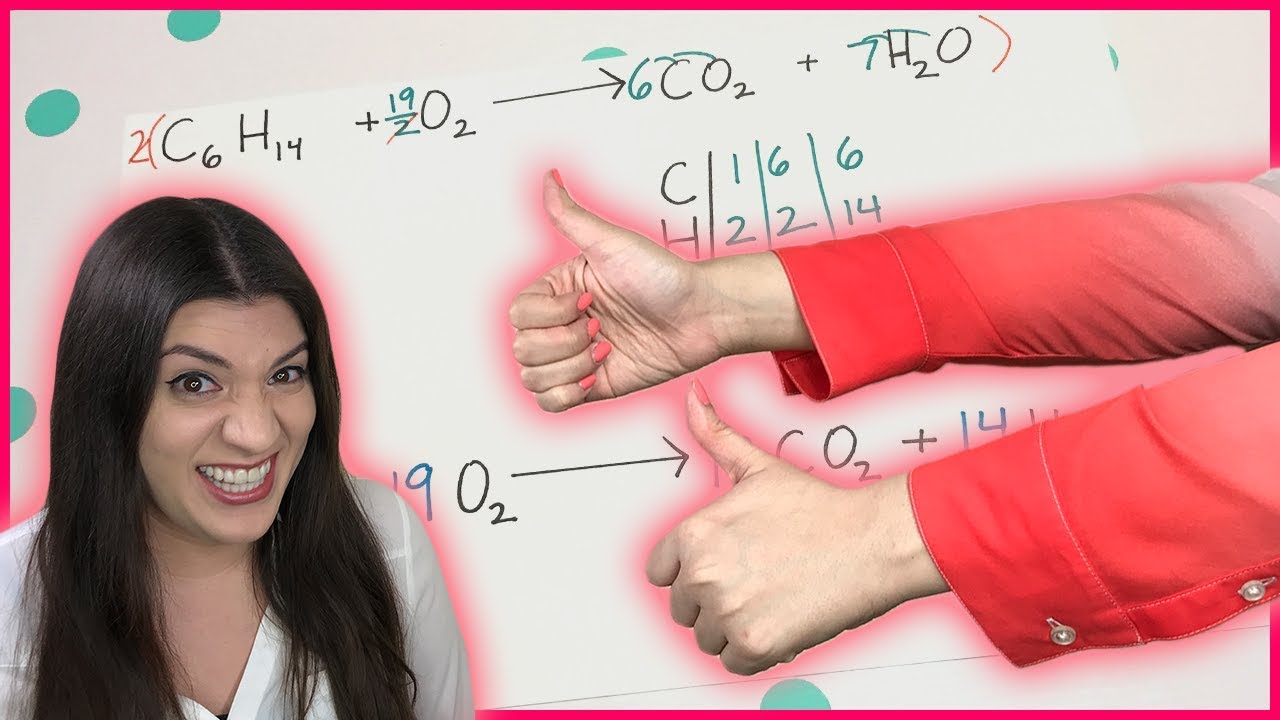
Introduction to Balancing Chemical Equations
TLDRThis educational video script offers a comprehensive guide on balancing chemical equations, focusing on various types of reactions including combustion, double replacement, and more. It emphasizes the importance of ensuring equal atom counts on both sides of the equation. The script walks viewers through step-by-step examples, starting with balancing carbon and hydrogen atoms, then adjusting oxygen atoms last. It covers specific cases like even-odd situations and provides strategies for whole number coefficients, concluding with a balanced equation for each scenario.
Takeaways
- 🔍 Balancing chemical equations is about ensuring the same number of atoms for each element on both sides of the reaction.
- 🔄 Start balancing by focusing on one element at a time, commonly carbon, then hydrogen, and finally oxygen.
- 📐 For the propane combustion reaction, coefficients are adjusted to ensure equal numbers of carbon, hydrogen, and oxygen atoms on both sides.
- ⚖️ In the butane combustion example, the equation is balanced by multiplying by two to avoid fractions and achieve whole numbers.
- 🛠️ When encountering an even-odd situation, multiplying by the least common multiple can help balance the equation.
- 🧩 In double replacement reactions, it can be easier to balance by considering polyatomic ions as single units.
- 💡 For the aluminum and hydrochloric acid reaction, multiplying by two rectifies the even-odd situation of hydrogen atoms.
- 🧪 In the gallium and copper bromide reaction, balancing involves adjusting coefficients to match bromine, copper, and gallium atoms on both sides.
- 🔗 In the I2 and F2 reaction, the least common multiple of fluorine atoms is used to balance the equation without leaving fractions.
- ⚛️ For the SO2 and O2 reaction, multiplying by two helps to balance the oxygen atoms and achieve a whole number balance.
- 🧪 In the sodium and sulfur reaction, coefficients are adjusted to ensure an equal number of sodium and sulfur atoms on both sides.
Q & A
What is the first step in balancing a combustion reaction according to the video?
-The first step is to balance the carbon atoms on both sides of the reaction.
After balancing the carbon atoms in a combustion reaction, what is the next step?
-The next step is to balance the hydrogen atoms.
In the combustion of propane (C3H8), how many oxygen molecules are needed to balance the equation?
-Five oxygen molecules (O2) are needed to balance the equation.
When balancing butane (C4H10) in a combustion reaction, why might you need to multiply the entire equation by two?
-Multiplying by two is necessary to avoid fractions and ensure all coefficients are whole numbers.
How do you handle an odd number of hydrogen atoms on one side and an even number on the other side of a reaction?
-You can rectify the situation by multiplying everything by two.
What is the balanced equation for the reaction of aluminum with hydrochloric acid?
-2 Al + 6 HCl → 2 AlCl3 + 3 H2.
What should you do if you encounter a fraction while balancing a chemical equation?
-Multiply the entire equation by the denominator of the fraction to convert all coefficients to whole numbers.
How do you balance a double replacement reaction more efficiently?
-It might be easier to view polyatomic ions (like PO4) as a whole unit and balance them as such.
What is the balanced equation for the reaction between sodium phosphate (Na3PO4) and magnesium chloride (MgCl2)?
-2 Na3PO4 + 3 MgCl2 → 6 NaCl + Mg3(PO4)2.
In a combustion reaction of ethanol (C2H5OH), how many oxygen molecules are needed to balance the equation?
-Three oxygen molecules (O2) are needed to balance the equation.
Outlines
🔍 Balancing Chemical Equations: Combustion Reactions
This paragraph introduces the concept of balancing chemical equations with a focus on combustion reactions. It uses propane as an example to demonstrate the step-by-step process of ensuring equal atoms on both sides of the equation. The video explains how to balance carbon and hydrogen atoms first, followed by oxygen, to achieve a balanced equation. It also touches on the method of dealing with fractions by multiplying the entire equation to achieve whole numbers.
🧩 Advanced Balancing Techniques: Even-Odd and Fractions
The second paragraph delves into more complex balancing scenarios, such as even-odd situations and the presence of fractions. It provides examples of reactions involving aluminum, gallium, iodine, and sulfur dioxide, illustrating how to rectify uneven atom counts and eliminate fractions by multiplying the entire equation or adjusting coefficients to achieve balanced equations with whole numbers.
🌐 Double Replacement Reactions: Simplifying the Process
This section discusses double replacement reactions, suggesting a simplified approach by treating complex molecules like phosphate as single units. It walks through the balancing of sodium phosphate and magnesium chloride reaction, emphasizing the importance of balancing the entire units first and then adjusting for individual atoms to achieve a balanced chemical equation.
🔄 Balancing Equations with Pure Elements and Fractions
The fourth paragraph addresses the balancing of equations that include pure elements and fractions. It uses the reaction between ammonia and oxygen gas as an example to show how to balance nitrogen and hydrogen atoms first, and then adjust for oxygen atoms last. The video demonstrates the process of eliminating fractions by multiplying the equation to ensure whole number coefficients.
🍾 Ethanol Combustion: A Step-by-Step Balancing Guide
The final paragraph provides a detailed guide on balancing the combustion reaction of ethanol. It outlines the step-by-step process of balancing carbon, hydrogen, and oxygen atoms, ensuring that the final equation reflects equal atom counts on both sides. The video concludes with a reminder to check the work and confirms the balanced state of the equation.
Mindmap
Keywords
💡Balancing Chemical Equations
💡Combustion Reaction
💡Carbon Atoms
💡Hydrogen Atoms
💡Oxygen Atoms
💡Stoichiometry
💡Aluminum
💡Hydrochloric Acid
💡Double Replacement Reaction
💡Sulfur
💡Ethanol
Highlights
Introduction to the process of balancing chemical equations, focusing on a combustion reaction involving propane and oxygen.
Explanation of the goal in balancing chemical equations: ensuring equal atoms on both sides of the reaction.
Step-by-step guide to balance carbon atoms first in the combustion reaction of propane.
Balancing hydrogen atoms in the propane combustion reaction by adjusting the coefficient in front of water (H2O).
Addressing the balancing of oxygen atoms by adjusting the coefficient in front of oxygen gas (O2).
Demonstration of balancing a second example reaction involving butane and oxygen.
Technique to avoid fractions in balanced equations by multiplying the entire equation by an appropriate factor.
Balancing the reaction between aluminum and hydrochloric acid, focusing on chlorine and hydrogen atoms.
Strategy for dealing with even-odd situations in chemical equations by multiplying to achieve whole numbers.
Balancing the reaction between gallium, copper bromide, and the formation of gallium bromide and copper metal.
Approach to balancing reactions with even-odd atom counts by finding the least common multiple.
Balancing the reaction between I2 and F2 to form IF7, emphasizing the importance of the least common multiple.
Balancing the reaction of SO2 with oxygen to form SO3 by adjusting coefficients to achieve whole numbers.
Consideration of balancing chemical equations with elements in their elemental form, such as sulfur in S8.
Balancing a double replacement reaction between sodium phosphate and magnesium chloride.
Strategy for balancing double replacement reactions by considering polyatomic ions as single units.
Balancing a second double replacement reaction involving potassium sulfate and aluminum chloride.
Approach to balancing a combustion reaction of ethanol, emphasizing the products CO2 and H2O.
Final summary of the video, highlighting the importance of balancing chemical equations for understanding chemical reactions.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: