Balancing Combustion Reactions
TLDRThe video script offers a comprehensive guide on balancing combustion reactions, typically involving hydrocarbons and oxygen. It emphasizes that complete combustion yields carbon dioxide and water as products. The script outlines a step-by-step process for balancing chemical equations, starting with carbon atoms, followed by hydrogen, and finally oxygen atoms. It also addresses scenarios where oxygen is limited, leading to the formation of carbon monoxide. Examples using methane, propane, butane, and more complex molecules like ethanol and propanol are provided to illustrate the balancing process. The video encourages viewers to practice by attempting to balance the combustion reaction of butane with oxygen gas, highlighting the importance of practice in mastering the topic.
Takeaways
- 🔥 **Combustion Reactions**: Typically involve hydrocarbons reacting with oxygen to produce carbon dioxide and water as products.
- ⚖️ **Balancing Equations**: Start by balancing carbon atoms, then hydrogen, and finally oxygen to ensure the equation is balanced.
- 📈 **Excess vs. Limited Oxygen**: With excess oxygen, you get CO2 and H2O, but with limited oxygen, you may also get CO.
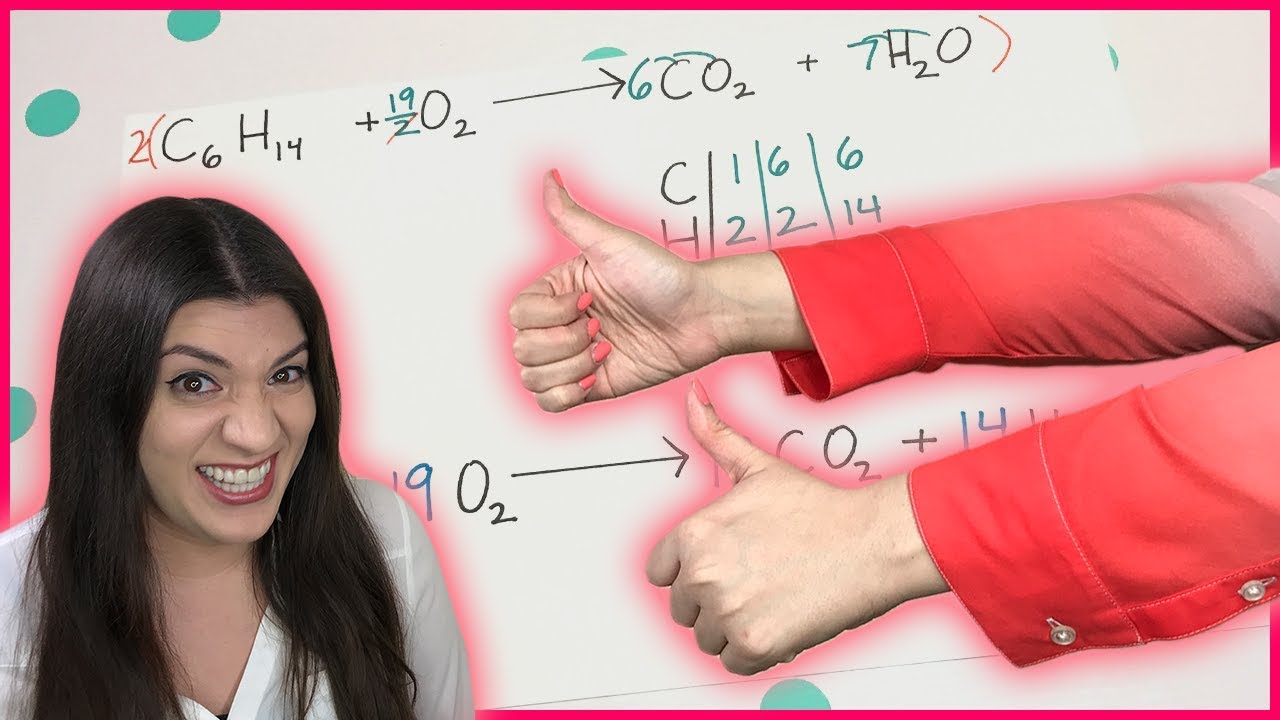
- 🛑 **Fractions in Balancing**: If you end up with a fraction when balancing oxygen atoms, multiply all coefficients by the denominator to eliminate the fraction.
- 🌐 **Oxygen Count**: Multiply the coefficient by the subscript of oxygen in CO2 and H2O to find the total number of oxygen atoms on the right side of the equation.
- 🔍 **Methane Combustion**: When methane (CH4) reacts with excess oxygen, the balanced products are CO2 and H2O.
- 🔄 **Propane Reaction**: Propane (C3H8) reacts with oxygen to produce 3 CO2 and 4 H2O molecules when balanced.
- 🛠️ **Ethane and Butane**: Ethane (C2H6) and butane (C4H10) are used as examples to demonstrate the balancing process for more complex hydrocarbons.
- 🎓 **Practice Makes Perfect**: The video encourages practice by suggesting the audience try balancing the combustion reaction of butane with oxygen.
- 🧪 **Additional Steps for Complex Molecules**: For molecules containing carbon, hydrogen, and oxygen, an additional step may be required to balance the oxygen atoms.
- ✅ **Double-Checking Work**: After balancing, it's important to double-check that the number of atoms for each element is equal on both sides of the equation.
Q & A
What are the typical products of a complete combustion reaction involving a hydrocarbon?
-The typical products of a complete combustion reaction involving a hydrocarbon are carbon dioxide (CO2) and water (H2O).
What happens if there is a limited supply of oxygen during a combustion reaction?
-If there is a limited supply of oxygen, the combustion reaction may produce other products such as carbon monoxide (CO) in addition to carbon dioxide.
How is the reaction between methane and excess oxygen balanced?
-The reaction is balanced by first balancing the carbon atoms, then the hydrogen atoms, and finally the oxygen atoms. The balanced equation is CH4 + 2O2 → CO2 + 2H2O.
What is the process for balancing the reaction between propane and oxygen gas?
-The process involves balancing the carbon atoms first, then the hydrogen atoms, and finally the oxygen atoms. The balanced equation is C3H8 + 5O2 → 3CO2 + 4H2O.
How do you balance a reaction when there is an odd number of oxygen atoms on the right side?
-If there is an odd number of oxygen atoms on the right side, you can balance it as a fraction and then multiply all coefficients and subscripts by a number to eliminate the fraction.
What is the balanced reaction for butane with oxygen gas?
-The balanced reaction is 2C4H10 + 13O2 → 8CO2 + 10H2O. This is achieved by balancing carbon, hydrogen, and then oxygen atoms, adjusting the coefficients accordingly.
How do you approach balancing a combustion reaction for a molecule that contains carbon, hydrogen, and oxygen?
-You follow similar steps as before: balance the carbon atoms, then the hydrogen atoms, and finally the oxygen atoms. If there's an odd number of oxygen atoms, use a fraction and then multiply to eliminate it.
What is the balanced reaction for propanol with oxygen gas?
-The balanced reaction is C3H8O + 9O2 → 6CO2 + 8H2O. This involves balancing carbon, hydrogen, and then using a fraction for oxygen before multiplying by 2 to eliminate the fraction.
Why is it important to balance the number of atoms for each element in a chemical reaction?
-Balancing the number of atoms for each element ensures that the law of conservation of mass is followed, meaning the number of atoms of each element is the same on both sides of the reaction.
What is the first step in balancing a combustion reaction in chemistry?
-The first step in balancing a combustion reaction is typically to balance the carbon atoms, as this ensures that the carbon present in the reactants is accounted for in the products.
How can one practice mastering the topic of balancing chemical equations?
-Practice can be achieved by working through various examples of combustion reactions, starting with simpler hydrocarbons and gradually moving to more complex molecules, ensuring to balance each element step by step.
What is the significance of the law of conservation of mass in the context of balancing chemical equations?
-The law of conservation of mass states that mass cannot be created or destroyed in a chemical reaction. This principle is fundamental when balancing chemical equations, as it ensures that the number of atoms for each element is equal on both sides of the equation.
Outlines
🔥 Combustion Reactions with Hydrocarbons
This paragraph introduces combustion reactions typically discussed in chemistry classes, focusing on hydrocarbons reacting with oxygen. It explains that complete combustion results in carbon dioxide and water when there is an excess of oxygen, while limited oxygen can lead to carbon monoxide. The paragraph also demonstrates how to balance chemical equations for complete combustion, using methane and propane as examples, and outlines the steps for balancing carbon, hydrogen, and oxygen atoms.
🧮 Balancing Equations and Fractions
The second paragraph delves into balancing more complex combustion reactions, including scenarios where fractions may arise. It guides through the process of multiplying coefficients and subscripts to eliminate fractions, using methane and butane as examples. The paragraph emphasizes the importance of practice in mastering the topic and provides a methodical approach to balancing carbon, hydrogen, and oxygen in chemical equations, even when dealing with pure elements like oxygen.
🌐 Advanced Combustion Reactions with Oxygen
The final paragraph tackles more challenging examples of combustion reactions, including molecules with carbon, hydrogen, and oxygen reacting with oxygen. It highlights an additional step in the balancing process when dealing with molecules that already contain oxygen. The paragraph provides a step-by-step guide to balancing carbon, hydrogen, and oxygen atoms, and demonstrates how to adjust for fractions by multiplying everything by a common factor to achieve a balanced equation. Propanol is used as an example to illustrate this process, ensuring that all atoms are balanced on both sides of the reaction.
Mindmap
Keywords
💡Combustion Reactions
💡Hydrocarbon
💡Oxygen
💡Carbon Dioxide
💡Water
💡Complete Combustion
💡Limited Supply of Oxygen
💡Balancing Chemical Equations
💡Methane
💡Propane
💡Ethanol
💡Propanol
Highlights
Introduction to combustion reactions, focusing on reactions involving hydrocarbons and oxygen.
Explanation of complete combustion reactions producing carbon dioxide and water with excess oxygen.
Discussion on incomplete combustion leading to carbon monoxide formation due to limited oxygen.
Example of balancing the combustion reaction of methane with oxygen.
Step-by-step guidance on balancing chemical equations by starting with carbon atoms.
Techniques for balancing hydrogen and oxygen atoms in chemical reactions.
Detailed balancing of a propane and oxygen combustion reaction.
Explanation of how to approach more complex combustion reactions with multiple carbon atoms.
Tutorial on calculating the necessary oxygen molecules to balance the reaction.
Illustrative example involving the combustion of ethene and the balancing challenges it presents.
Description of using fractions in balancing reactions and how to eliminate them by doubling coefficients.
Instructional example of reacting butane with oxygen, demonstrating steps to balance the equation.
Introduction to reactions involving compounds with carbon, hydrogen, and oxygen, like ethanol.
Balancing complex chemical equations involving additional elements such as oxygen within organic molecules.
Detailed walkthrough of a propanol combustion reaction, showcasing the balancing of carbons, hydrogens, and oxygens.
Transcripts
Browse More Related Video

Predicting Products | Combustion Reactions

How To Balance Combustion Reactions
Balancing Chemical Equations With Fractions | How to Pass Chemistry

How To Balance Chemical Equations

GCSE Chemistry - Alkanes: properties & combustion #52

Balancing more complex chemical equations | Chemical reactions | High school chemistry |Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: