Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (17 of 92) How to Use Schrod. Eqn: 2
TLDRThe video script delves into the relationship between the Schrödinger equation and the wave-like behavior of particles in physical structures, such as atoms and potential wells. It explains how particles, like electrons, form standing wave patterns corresponding to their energy levels and orbits, with quantized energy states. The importance of boundary conditions and physical dimensions in determining wave functions and energy levels is highlighted, emphasizing the quantized nature of energy and the role of the Schrödinger equation in predicting particle probabilities and behaviors.
Takeaways
- 📌 The Schrodinger equation's structure and segments were previously discussed, and now we explore how it helps in understanding a particle's wave equation.
- 🌐 Particles in real-life situations are often confined, such as in atomic orbits or by potential energy barriers, leading to standing wave-like behavior.
- 🌊 Particles like electrons exhibit probabilistic wave-like behavior, where the probabilities of their existence resemble standing waves.
- 🔄 When particles are limited in physical structures, they appear to exist only as standing waves, with their energy quantized into specific levels.
- ⚛️ The wavelength of a particle in an orbit, like an electron in a hydrogen atom, is related to the distance of one orbit around the nucleus.
- 🏢 In a potential well or box, particles can only exist as standing waves without destructive interference, which is a requirement for their presence in a location.
- 📈 The time-independent Schrödinger equation is key to determining the physical situation of a particle, its energy, and its wavefunction.
- 🔢 The energy of particles is quantized, meaning they can only exist in specific locations with specific wave representations, leading to quantum jumps between energy levels.
- 🌟 Understanding a particle's geometry, boundary conditions, and physical conditions is crucial for determining its potential, kinetic, and total energy, and thus its wave equation.
- 📊 The Schrödinger equation, combined with the physical conditions of a particle, allows us to derive the wave equation and probability functions that describe a particle's behavior.
Q & A
What is the main topic of the video?
-The main topic of the video is understanding how the Schrödinger equation helps in determining the wave equation of a particle, particularly in real-life situations such as orbits of atoms or within potential wells.
How does the particle's appearance relate to standing waves?
-Particles, while not waves themselves, exhibit probabilities that look like waves. When confined in physical structures, these probabilities resemble standing waves, with the particle's behavior mirroring the characteristics of a standing wave.
What is the significance of the wavelength in relation to an electron's orbit in a hydrogen atom?
-The wavelength of the wave associated with an electron in an orbit is equal to the distance of one orbit around the nucleus. This relationship is crucial in understanding the quantized energy levels of electrons in atoms.
How does the potential well model relate to a particle's existence as a standing wave?
-In a potential well or box model, a particle can only exist if its wave-like probability function resembles a standing wave. If the particle's path does not align with a standing wave pattern, destructive interference occurs, and the particle cannot exist in that state.
What does the time-independent Schrödinger equation help us determine?
-The time-independent Schrödinger equation allows us to determine the physical situation of a particle, such as its energy levels, potential energy, kinetic energy, and the wave function based on the particle's geometric and boundary conditions.
How are energy levels quantized?
-Energy levels are quantized, meaning a particle cannot exist in an infinite number of locations. It can only exist in specific locations with specific wave function representations, which correspond to distinct energy levels.
What is the role of boundary conditions in determining a particle's wave function?
-Boundary conditions are crucial in determining a particle's wave function as they define the physical limits within which the particle can exist. These conditions help establish the standing wave pattern and the associated probabilities.
How does the geometry of a particle's location influence the wave equation?
-The geometry of a particle's location, including its physical dimensions and boundary conditions, is essential for determining the potential and kinetic energy, which in turn allows us to find the wave equation using the Schrödinger equation.
What happens when a particle's orbit is equal to two and a half wavelengths?
-When a particle's orbit equals two and a half wavelengths, destructive interference occurs, preventing the particle from existing in that state because the wave-like probability function does not support a standing wave pattern.
What is the significance of the Schrödinger equation in quantum mechanics?
-The Schrödinger equation is fundamental in quantum mechanics as it provides a mathematical framework to describe the quantum state of a physical system. It allows us to calculate the probability functions and predict the behavior of particles at the quantum level.
Outlines
🌟 Understanding the Schrodinger Equation and Wave Function
This paragraph delves into the relationship between the Schrodinger equation and the wave function of a particle. It explains how particles, such as electrons, can be found in real-life situations like orbits of atoms or confined by potential energy. The concept of standing waves is introduced to describe the probabilistic nature of particles' existence, drawing a parallel between the wavelengths of these waves and the distances particles can exist in, such as electron orbits in atoms. The importance of boundary conditions and physical dimensions in determining the energy levels and wave functions of particles is emphasized, highlighting the quantization of energy and the discrete nature of particle existence in specific locations.
📈 Applying the Schrodinger Equation to Find Wave Equations
The second paragraph focuses on the practical application of the Schrodinger equation to derive wave equations for particles. It discusses how understanding the physical conditions and boundary constraints of a particle's environment is crucial for determining its potential, kinetic, and total energy. The paragraph explains that these factors are essential for identifying the wave function and probability distribution of a particle's existence. The concept of quantum jumps between energy levels is introduced, and the idea of using the time-independent Schrödinger equation to find wave functions is explored. The paragraph concludes by encouraging viewers to stay tuned for further examples of these concepts in action.
Mindmap
Keywords
💡Schrodinger Equation
💡Wave Equation
💡Standing Wave
💡Quantum Mechanics
💡Probability Function
💡Potential Energy
💡Boundary Conditions
💡Quantization
💡Energy Levels
💡Destructive Interference
💡Potential Well
Highlights
Understanding the structure of the Schrodinger equation and its segments.
Exploring how the time-independent linear equation helps in determining the wave equation of a particle.
Particles in real-life situations, such as being bound in orbits of atoms or confined by physical dimensions.
The concept of particles existing as standing waves, despite not being waves themselves.
The relationship between the wavelength of a particle's wave and the distance of its orbit around the nucleus.
The association between the path of particles and the distance they're allowed to be in, specifically in orbits.
The quantization of energy levels, meaning particles can only exist in specific locations with specific wave representations.
Quantum jumps between energy levels where particles can exist.
Using the physical situation of a particle, such as being in an orbit or a potential well, to derive expressions for energy.
The importance of physical dimensions and boundary conditions in determining the wave equation.
The role of geometry and physical conditions in determining the potential and kinetic energy of a particle.
How the limitations of a particle's environment contribute to understanding its potential energy, kinetic energy, and total energy.
The process of using the Schrodinger equation to find the wave equation and probability function of a particle.
The necessity of considering the physical conditions and boundary conditions to determine the wave equation.
The potential for destructive interference if a particle's orbit does not correspond to an integer number of wavelengths.
The practical application of these concepts in understanding the behavior of particles like electrons in atoms.
Transcripts
Browse More Related Video
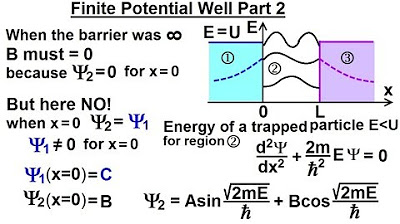
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (33 of 92) Finite Potential Well Part 2
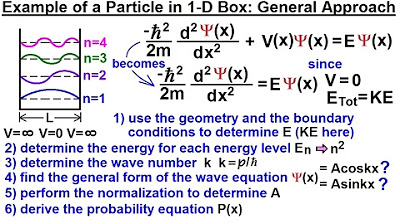
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (18 of 92) Particle in 1-D Box: Gen. Appr.
23. Quantum Mechanics V: Particle in a Box
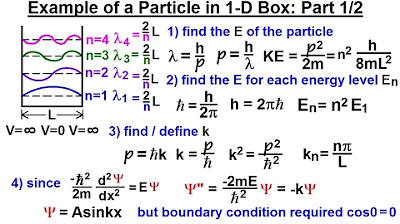
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (19 of 92) Particle in 1-D Box: Example 1/2
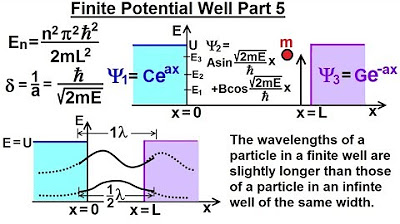
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (36 of 92) Finite Potential Well Part 5
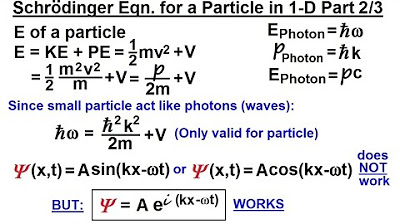
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (6 of 92) The Schrodinger Eqn. in 1-D (2/3)
5.0 / 5 (0 votes)
Thanks for rating: