How to Use Each Gas Law | Study Chemistry With Us
TLDRThis video transcript focuses on understanding and applying gas laws, emphasizing the importance of unit conversion, particularly to Kelvin for temperature. It outlines the key gas laws, including Boyle's, Charles', Avogadro's, the combined gas law, and the ideal gas law, and provides a step-by-step guide on how to select the appropriate law for solving problems. The transcript also highlights the significance of memorizing formulas and unit conversions for successful problem-solving in exams.
Takeaways
- 📚 Always convert temperatures to Kelvin for gas laws involving temperature.
- 🌡️ To convert Celsius to Kelvin, add 273 to the Celsius value.
- 📊 Know the common pressure units: 1 atmosphere = 760 torr = 760 mmHg.
- 🔍 Identify the correct gas law by analyzing the given variables and what you're solving for.
- 📐 Boyle's Law relates pressure and volume (ignores temperature and moles).
- 🔄 Charles's Law compares volume and temperature (ignores pressure and moles).
- ⚖️ Avogadro's Law deals with volume and moles (ignores pressure and temperature).
- 🌀 Combined Gas Law combines pressure, volume, and temperature.
- 🌐 Ideal Gas Law encompasses pressure, volume, moles, and temperature with the gas constant R.
- 🤝 Memorize the formulas and unit conversions to solve gas law problems efficiently.
- 📈 For significant figures, consider the smallest unit value from the multiplication or division process.
Q & A
What is the key fact to remember about temperature units in gas laws?
-For every gas law that includes temperature, the temperature must be in Kelvin. If the temperature is given in Celsius, it should be converted to Kelvin by adding 273.
How do you convert Celsius to Kelvin?
-To convert Celsius to Kelvin, add 273 to the Celsius temperature value.
What are the common units for pressure and their conversions?
-Common units for pressure include atmospheres (atm), torr, and millimeters of mercury (mmHg). One atmosphere is equal to 760 torr or 760 mmHg.
Which gas law relates pressure and volume only?
-Boyle's Law relates pressure and volume only, ignoring temperature and moles.
Which gas law compares volume and temperature?
-Charles's Law compares volume and temperature, holding pressure and moles constant.
What is the combined gas law?
-The combined gas law compares pressure, volume, and temperature, taking into account the number of moles and the gas constant.
What is the ideal gas law and what does it compare?
-The ideal gas law compares pressure, volume, temperature, and moles, with the gas constant being a known value provided during the problem-solving process.
How does the script suggest determining which gas law to use for a problem?
-The script suggests writing down the given values and what you are trying to find, as this will indicate which formula to use based on the variables involved.
What is the significance of the gas constant (R) in the ideal gas law?
-The gas constant (R) is a known value that relates the molar volume of a gas to its temperature and pressure. It ensures the units are consistent in the ideal gas law equation.
How many significant figures should be used when rounding the final answer in a gas law calculation?
-The number of significant figures to use when rounding the final answer depends on the smallest number of significant figures among the values plugged into the formula.
What is the importance of unit conversion in gas law calculations?
-Unit conversion is crucial in gas law calculations to ensure that all units are consistent and compatible with the formula being used, especially when dealing with the ideal gas law and its specific units (liters, atmospheres, moles, and Kelvin).
Outlines
📚 Introduction to Gas Laws
The video begins with an introduction to gas laws, emphasizing their importance in understanding various scientific phenomena. The focus is on the mathematical aspect of these laws, as they form the majority of the exam content. The instructor plans to cover the different formulas and how to identify which law to apply based on word problems. The key fact highlighted is the necessity of using Kelvin when temperature is involved in the formulas. Additionally, the importance of unit conversion, particularly from Celsius to Kelvin, is stressed to avoid errors in calculations.
🔍 Applying Gas Laws: Boyle's and Charles' Laws
This section delves into the application of Boyle's and Charles' laws. Boyle's law is discussed in the context of pressure and volume, ignoring temperature and moles. Charles' law, on the other hand, compares volume and temperature. The instructor uses a problem-solving approach to demonstrate how to identify the correct formula based on the given variables and how to convert temperatures from Celsius to Kelvin. The process of solving for the new volume when pressure remains constant is outlined, highlighting the importance of unit consistency and the steps to arrive at the final answer.
📈 Gas Law Formulas and Problem Solving
The paragraph outlines the main gas laws that will be covered, including Boyle's, Charles', Avogadro's, the combined gas law, and the ideal gas law. The instructor provides a cheat sheet to help viewers determine which gas law to use in different scenarios. A detailed example is given, showing how to work through a problem involving a change in volume due to temperature change, while pressure remains constant. The importance of converting temperatures to Kelvin and the relationship between different units of pressure are reiterated, with a step-by-step guide on how to solve the problem and the significance of significant figures in the final answer.
🌡️ Temperature Conversion and Unit Consistency
This paragraph emphasizes the need to convert temperatures to Kelvin when dealing with gas laws that involve temperature. The instructor provides a clear method for converting Celsius to Kelvin and stresses the importance of unit consistency, especially when dealing with pressure units. The video continues with a problem that requires finding the new pressure when a gas is compressed, using the correct formula that relates volume and pressure. The process of plugging in values, converting units, and solving for the unknown is thoroughly explained, with a focus on arriving at the correct answer in the appropriate units.
📊 Combined Gas Law and Ideal Gas Law
The combined gas law, which incorporates volume, pressure, and temperature, is introduced as a more complex formula. The ideal gas law is highlighted as the most comprehensive, involving all four variables. The instructor explains how to use these laws by identifying the given and sought variables, and then applying the appropriate formula. A problem is solved step by step, demonstrating how to handle multiple variables and units, and the importance of using the correct gas constant units. The process of solving for volume, considering moles, temperature, and pressure, is detailed, with an emphasis on the final answer's unit and significant figures.
🎓 Final Thoughts and Encouragement
In the concluding part of the video, the instructor reiterates the importance of understanding and memorizing the gas laws for the final exam. The video provides a comprehensive overview of the different gas laws, their applications, and the problem-solving process. The instructor encourages viewers to practice and review the material, ensuring they are comfortable with the concepts and formulas. The aim is to make the complex topic of gas laws more accessible and manageable, boosting confidence in tackling exam questions.
Mindmap
Keywords
💡Gas Laws
💡Boyle's Law
💡Charles's Law
💡Avogadro's Law
💡Combined Gas Law
💡Ideal Gas Law
💡Unit Conversion
💡Significant Figures
💡Problem Solving
💡Gas Constants
Highlights
Understanding which gas law to use and how to select one for problem-solving is crucial.
For formulas involving temperature, ensure units are in Kelvin to avoid errors.
The conversion from Celsius to Kelvin is done by adding 273 to the Celsius value.
When dealing with pressure, be aware of different units such as atmospheres and torr, and know how to convert between them.
Boyle's Law relates pressure and volume, ignoring temperature and moles.
Charles's Law compares volume and temperature, holding pressure and moles constant.
Ampère's Law, also known as Avogadro's Law, relates volume and moles while keeping pressure and temperature constant.
The Combined Gas Law is tricky as it combines pressure, volume, and temperature, and requires understanding of moles and the gas constant.
The Ideal Gas Law is comprehensive, comparing pressure, volume, temperature, and moles, with the gas constant provided.
When solving problems, start by identifying given values and what you're solving for to determine the correct formula.
Always convert temperatures to Kelvin when they are part of the problem.
Unit conversion is essential; for example, knowing that 1 atmosphere equals 760 torr is important for pressure calculations.
Memorizing the gas laws and their formulas is key to success in solving gas law problems.
When working with gas laws, pay attention to the units required by the problem and ensure they match the gas constant's units.
Significant figures play a role in rounding your final answers, and you should round based on the smallest number of significant figures in your calculation.
For complex problems involving multiple variables, use the Combined Gas Law or Ideal Gas Law to account for all factors.
In the absence of specific unit requirements, the default unit for volume in gas law calculations is liters.
The gas constant's units (liters, atmospheres, moles, and Kelvin) facilitate seamless calculations without the need for unit conversion.
When solving gas law problems, always round your final answer to the appropriate number of significant figures based on the input values.
Transcripts
Browse More Related Video
9.2 Gas Laws including the Ideal Gas Law | High School Chemistry

Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion

Step by Step Gas Stoichiometry - Final Exam Review
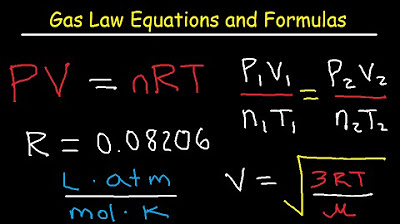
Gas Laws - Equations and Formulas
Ideal Gas Problems: Crash Course Chemistry #13

Kinetic Molecular Theory and the Ideal Gas Laws
5.0 / 5 (0 votes)
Thanks for rating: