Ideal Gas Problems: Crash Course Chemistry #13
TLDRThis video explains the ideal gas law, which combines Boyle's, Charles's, and Avogadro's gas laws into one equation to describe the behavior of gases. It assumes gases have no volume and no intermolecular attractions. After reviewing the individual laws and how they relate, calculations are done to find the volume of a gas at STP and the pressure changes inside the Hindenburg. The limitations of the ideal gas law are discussed, along with a demonstration showing that increased gas pressure causes increased temperature.
Takeaways
- 😀 The ideal gas law combines Boyle's law, Charles' law, and Avogadro's law into one equation using a universal gas constant R.
- 😧 The ideal gas law makes assumptions that gas particles have no volume and don't attract each other, which isn't completely realistic.
- 🤓 Using the ideal gas law, you can calculate the volume 1 mole of a gas occupies at standard temperature and pressure.
- 🌡️ Increasing gas pressure while keeping volume constant leads to an increase in temperature.
- 💥 The Hindenburg airship was filled with hydrogen gas which is flammable and contributed to its destruction.
- 🧮 You can use the ideal gas law to calculate the number of moles of hydrogen gas the Hindenburg contained.
- 🔬 The ideal gas law allows comparing how much more lift hydrogen provided over helium for the Hindenburg.
- 📉 Changes in temperature or pressure lead to changes in volume or moles needed to keep the ideal gas law balanced.
- ☄️ Rapidly compressing air in a chamber causes its temperature to rise enough to ignite material like cotton.
- 🎓 The assumptions the ideal gas law makes work fairly well under normal conditions but break down at high pressures or low temperatures.
Q & A
What gas law relates pressure and volume, assuming temperature is constant?
-Boyle's law states that for a gas at constant temperature, pressure and volume are inversely proportional.
Who combined the three previous gas laws into the ideal gas law equation?
-Dmitri Mendeleev combined Boyle's law, Charles' law and Avogadro's law into the ideal gas law equation.
What assumptions does the ideal gas law make about gas particles?
-The ideal gas law assumes gas particles have no volume and do not attract each other.
What is the reason the Hindenburg is so well remembered today?
-The Hindenburg is well remembered for catching fire and crashing in New Jersey in 1937, killing 36 people.
Why did the Hindenburg use highly flammable hydrogen instead of helium?
-Hydrogen was much cheaper than helium at the time, and also provided more lift for the airship.
How can increased gas pressure lead to increased temperature?
-When a gas is compressed, the molecules are pushed closer together and their kinetic energy increases, resulting in a temperature rise.
What happens to the pressure of a fixed amount of gas if the temperature rises?
-Charles' law states that for a fixed amount of gas, pressure and temperature are directly proportional. So if temperature rises, pressure will also rise.
What causes the ideal gas law to break down under certain conditions?
-At high pressures or low temperatures, the gas molecules are very close together so they take up significant volume and attract each other more.
What was the Nazi propaganda name they wanted to give the Hindenburg?
-The Nazi propaganda minister Joseph Goebbels wanted to name the airship "The Hitler," but the Germans refused.
What happens when you compress air suddenly in a fire piston?
-The sudden compression causes a sharp rise in temperature that can ignite a piece of cotton, proving the relationship between pressure and temperature.
Outlines
😊 Ideal Gas Law Overview and Calculations
This paragraph introduces the ideal gas law, explaining that it combines Boyle's law, Charles' law, and Avogadro's law into one equation. It reviews each historical law briefly and explains how Dmitri Mendeleev combined them by calculating a universal gas constant R. It notes that the ideal gas law makes assumptions about particles not taking up space and not attracting each other. The paragraph then demonstrates using the ideal gas law to calculate the volume of 1 mole of gas at standard temperature and pressure.
😮 Using the Ideal Gas Law for the Hindenburg Disaster
This paragraph examines the 1937 Hindenburg disaster, providing background on the airship. It calculates the number of moles of hydrogen gas aboard the Hindenburg using the ideal gas law and data about its gas cells. It compares how much more the ship could carry using hydrogen instead of helium. Finally, it calculates how much the internal pressure would increase when moving from cooler Germany to warmer New Jersey.
🔥 Demonstrating the Ideal Gas Law with Fire
This closing paragraph demonstrates the ideal gas law using a fire piston to suddenly increase air pressure and heat inside to ignite cotton. It reinforces key learnings about how the ideal gas law combines historical gas laws and assumptions. It thanks viewers and credits the team involved in creating the video.
Mindmap
Keywords
💡ideal gas law
💡STP
💡atmospheric pressure
💡hydrogen
💡helium
💡pressure
💡volume
💡temperature
💡amount
💡corrections
Highlights
The ideal gas law assumes particles have no size and don't attract, unlike real gases and people.
Boyle's, Charles's, and Avogadro's gas laws describe the behavior of gases under different conditions.
Mendeleev combined the three laws into the ideal gas law equation using a universal gas constant R.
The ideal gas law works well under normal conditions but breaks down at high pressure, low temperature, or density.
Standard temperature and pressure (STP) is 0°C and 100 kPa, used as a baseline to compare gas behaviors.
At STP, 1 mole of an ideal gas occupies 22.7 liters of volume based on the ideal gas law equation.
The Hindenburg zeppelin was nearly as big as a cruise ship and used hydrogen for lift, which was cheaper but flammable.
The Hindenburg started with 9 million moles of hydrogen gas, providing 18 metric tons more lift than helium.
In warmer New Jersey, the Hindenburg's internal pressure increased to 103 kPa from 100 kPa in Germany.
Adiabatic compression of air in the fire piston causes a sudden extreme rise in temperature high enough to ignite cotton.
Taking up space and being attracted to others cause problems for real gases, just like for people.
Mendeleev unified gas laws like he organized elements into the periodic table.
The Hindenburg used hydrogen instead of helium for economic reasons despite greater flammability.
The ideal gas law works under normal conditions but fails at high pressure, low temperature or density.
Adiabatic compression can generate enough heat to ignite material like cotton.
Transcripts
Browse More Related Video
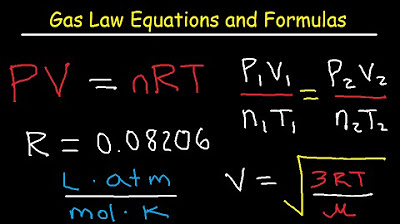
Gas Laws - Equations and Formulas

Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion
Kinetic Molecular Theory of Gases - Practice Problems
9.2 Gas Laws including the Ideal Gas Law | High School Chemistry

Kinetic Molecular Theory and the Ideal Gas Laws
Temperature: Crash Course Physics #20
5.0 / 5 (0 votes)
Thanks for rating: