Kinetic Molecular Theory and the Ideal Gas Laws
TLDRProfessor Dave introduces the concept of ideal gases, explaining their definition and the simplifying assumptions made for ease of calculations. He discusses the four key variables of pressure, temperature, volume, and moles, and how they interrelate through Boyle's Law, Charles's Law, and the Ideal Gas Law. The video also touches on the importance of using the Kelvin scale for temperature calculations and Avogadro's Law, emphasizing how these principles allow for the calculation of any variable when the others are known.
Takeaways
- 🌟 Ideal gases are theoretical constructs that simplify the behavior of real gases under certain assumptions.
- 🔵 The two fundamental assumptions for ideal gases are that particles are dimensionless points in random motion and they interact only through elastic collisions.
- 📏 Four key variables describe ideal gases: pressure (force on container), temperature (heat energy and particle speed), volume (container size), and moles (number of particles).
- 📈 Boyle's Law states that pressure and volume are inversely proportional (P1V1 = P2V2) for a gas at constant temperature and moles.
- 🌡️ Charles's Law indicates that volume and temperature are directly proportional for a gas at constant pressure and moles.
- 🌍 The Kelvin scale is an absolute temperature scale used in gas laws, with 0 K representing absolute zero.
- 🔄 To convert Celsius to Kelvin, add 273; to convert Kelvin to Celsius, subtract 273.
- 💡 Avogadro's Law asserts that equal volumes of gases at the same temperature and pressure contain the same number of molecules, with one mole occupying 22.4 liters at STP.
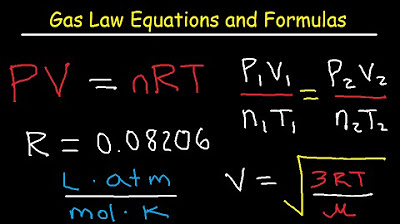
- 📝 The Ideal Gas Law combines all four variables with the gas constant (R) to calculate the properties of a gas when three of the four variables are known.
- 🔢 With the Ideal Gas Law, one can solve for the fourth variable if three are given, or use Boyle's, Charles's, or Avogadro's Laws to find changes in conditions.
Q & A
What is the definition of a gas according to the script?
-A gas is the phase of matter where atoms of a substance are in motion and fill their container.
What are the two simplifying assumptions made about gases when discussing ideal gases?
-The two assumptions are that particles in the gas are dimensionless points in random motion, and the identity of the gas is irrelevant; and that the particles don't interact apart from elastic collisions.
What are the four variables that are important when examining an ideal gas?
-The four variables are pressure, temperature, volume, and moles.
How is pressure defined in the context of an ideal gas?
-Pressure is the force the gas is exerting on its container, or how much the particles are hitting the sides.
What is Boyle's Law and how is it expressed?
-Boyle's Law states that pressure and volume are inversely proportional. It is expressed as P1V1 equals P2V2.
What does Charles's Law state about the relationship between volume and temperature?
-Charles's Law states that volume and temperature are directly proportional. If one doubles, the other must double to keep the pressure constant.
Why is the Kelvin scale used instead of Celsius when dealing with gas laws?
-The Kelvin scale is used because it is an absolute temperature scale starting at absolute zero, which avoids mathematical issues with negative or zero temperatures.
How can you convert Celsius to Kelvin?
-To convert Celsius to Kelvin, you add 273 to the Celsius temperature.
What does Avogadro's Law state?
-Avogadro's Law states that equal volumes of gas at the same temperature and pressure contain the same number of molecules, and that one mole of ideal gas occupies 22.4 liters at standard temperature and pressure.
What is the Ideal Gas Law and how does it relate to the other gas laws?
-The Ideal Gas Law is an equation that combines the principles of Boyle's Law, Charles's Law, and Avogadro's Law. It relates pressure, volume, temperature, and moles with the gas constant (R).
How can you use the Ideal Gas Law to find unknown variables?
-If you know three of the four variables (pressure, volume, temperature, and moles), you can use the Ideal Gas Law to calculate the fourth variable.
Outlines
📚 Introduction to Ideal Gases
This paragraph introduces the concept of ideal gases, starting with a definition of a gas as a phase of matter where atoms are in motion and fill their container. It discusses the simplifying assumptions made about gases, such as considering the particles as dimensionless points in random motion and the lack of interaction between particles except for elastic collisions. These assumptions, while not entirely true, simplify the mathematics and often yield accurate results. The paragraph also outlines the four key variables used when examining an ideal gas: pressure, temperature, volume, and moles, explaining their relevance and interdependence.
Mindmap
Keywords
💡Ideal Gas
💡Boyle's Law
💡Charles's Law
💡Kelvin Scale
💡Avogadro's Law
💡Ideal Gas Law
💡Gas Constant (R)
💡Moles
💡Pressure
💡Temperature
💡Volume
Highlights
Definition of an ideal gas as a phase of matter where atoms are in motion and fill their container.
Simplifying assumptions for gases include particles being dimensionless points in random motion and non-interacting except for elastic collisions.
The identity of the gas particles is irrelevant in the ideal gas model.
Four variables to discuss when examining an ideal gas: pressure, temperature, volume, and moles.
Boyle's law states that pressure and volume are inversely proportional (P1V1 = P2V2) for a gas at constant temperature and moles.
Charles's law indicates that volume and temperature are directly proportional for a gas at constant pressure and moles.
The Kelvin scale is used for temperature calculations, with 0 Kelvin representing absolute zero.
To convert Celsius to Kelvin, add 273; to convert Kelvin to Celsius, subtract 273.
Avogadro's law states that equal volumes of gas at the same temperature and pressure contain the same number of molecules.
One mole of an ideal gas occupies 22.4 liters at standard temperature and pressure, regardless of the gas's identity.
The ideal gas law combines all four variables with the gas constant R, allowing for calculations involving pressure, volume, temperature, and moles.
The ideal gas law is useful for determining the value of any variable when the other three are known.
Initial and final conditions can be used with the ideal gas law or other gas laws to solve for unknown variables.
The gas constant R has different values depending on the units used, with a predominant value provided for calculations.
Understanding the ideal gas law and its related laws is crucial for various scientific and engineering applications.
The relationship between the variables in an ideal gas can be used to predict the behavior of gases under different conditions.
These gas laws provide a foundation for the study of thermodynamics and the properties of matter.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: