Gibbs free energy and spontaneity | Chemistry | Khan Academy
TLDRThis video script explores the concept of spontaneity in chemical reactions, focusing on the role of enthalpy (ΔH), entropy (ΔS), and temperature. It explains how exothermic reactions (ΔH < 0) tend to be spontaneous, while endothermic reactions (ΔH > 0) require energy. The script also delves into the impact of entropy changes and introduces the Gibbs free energy (ΔG) formula as a predictor of spontaneity.
Takeaways
- 🔥 The change in enthalpy (ΔH) at constant pressure is equal to the heat added to the system.
- 🌡️ A negative ΔH indicates an exothermic reaction, where energy is released, while a positive ΔH signifies an endothermic reaction, where energy is absorbed.
- 🌌 Spontaneity of a reaction is not solely determined by enthalpy; entropy (ΔS) and temperature also play crucial roles.
- 🤔 High kinetic energy from high temperatures can counteract the tendency for reactions to proceed in the direction of lower potential energy.
- 🔄 An increase in entropy (ΔS > 0) generally favors spontaneity, as it corresponds to a system accessing more microstates.
- ♨️ At low temperatures, the release of energy (exothermic process) tends to drive a reaction to be spontaneous due to the dominance of enthalpy over entropy.
- 🔥 High temperatures can make a reaction that decreases entropy (ΔS < 0) spontaneous, due to the high kinetic energy overcoming the enthalpy change.
- 🤹♂️ The likelihood of a reaction occurring spontaneously depends on the interplay between enthalpy, entropy, and temperature.
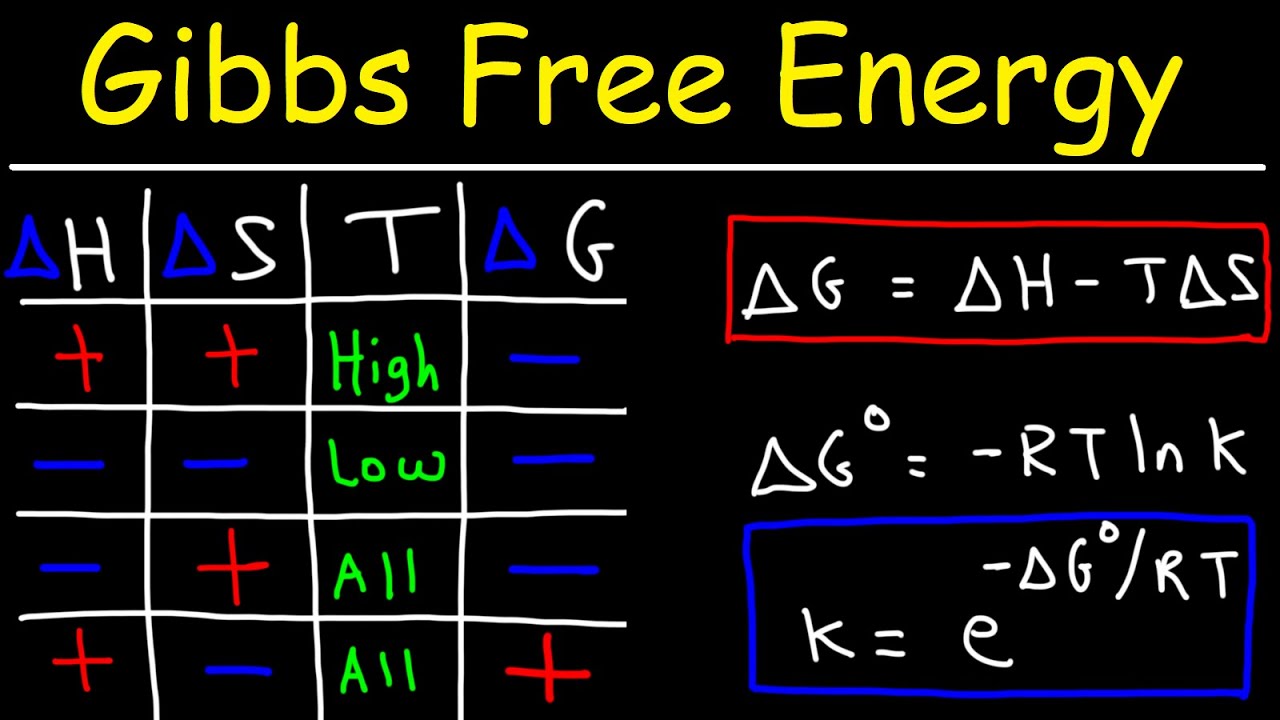
- 📉 The formula ΔG = ΔH - TΔS predicts spontaneity, where ΔG is the change in Gibbs free energy. A negative ΔG indicates a spontaneous process.
- 🔮 The concept of spontaneity does not address the rate of a reaction, only whether it will occur without external influence.
- 🧩 The video aims to build an intuitive understanding of the factors influencing spontaneity before presenting the Gibbs free energy equation more formally.
Q & A
What is the relationship between change in enthalpy and heat added to a system at constant pressure?
-The change in enthalpy (∆H) at constant pressure is equal to the heat added to the system. If ∆H is negative, it indicates that the system is releasing heat, and if it is positive, the system is absorbing heat.
How does the sign of ∆H indicate whether a reaction is exothermic or endothermic?
-A negative ∆H indicates that the reaction is exothermic, meaning it releases energy. Conversely, a positive ∆H signifies an endothermic reaction, where the system absorbs energy.
What is the significance of ∆S (change in entropy) in determining the spontaneity of a reaction?
-∆S represents the change in entropy, which is a measure of disorder. An increase in entropy (positive ∆S) generally favors spontaneity, while a decrease in entropy (negative ∆S) makes a reaction less likely to occur spontaneously.
How does temperature affect the spontaneity of a reaction?
-Temperature plays a crucial role in determining spontaneity. At high temperatures, entropy changes (∆S) become more significant, potentially overriding the enthalpy change (∆H). At low temperatures, the enthalpy change dominates the spontaneity decision.
What is the formula for predicting spontaneity in a reaction?
-The formula for predicting spontaneity is given by ∆G = ∆H - T∆S, where ∆G is the change in Gibbs free energy, T is the temperature in Kelvin, and ∆S is the change in entropy. A negative ∆G indicates a spontaneous reaction.
Why is ∆G (change in Gibbs free energy) considered a better indicator of spontaneity than just ∆H?
-∆G incorporates both the enthalpy change (∆H) and the entropy change (∆S), weighted by temperature. This makes it a more comprehensive measure of spontaneity, as it considers both energy changes and disorder changes in a system.
What happens when a reaction has a negative ∆H and a positive ∆S?
-A reaction with a negative ∆H (exothermic) and a positive ∆S (increase in entropy) is highly likely to be spontaneous, as both factors contribute to a negative ∆G, which is a favorable condition for spontaneity.
In what scenario might a reaction with a negative ∆H not be spontaneous?
-A reaction with a negative ∆H might not be spontaneous if the entropy change (∆S) is negative and the temperature is high enough such that the T∆S term outweighs the negative ∆H, resulting in a positive ∆G.
How does the number of particles in a system relate to its entropy?
-The entropy of a system is related to the number of particles and the states they can occupy. More particles generally lead to higher entropy, as there are more possible configurations and states the system can exist in.
What is the role of kinetic energy in the spontaneity of a reaction at high temperatures?
-At high temperatures, the kinetic energy of particles is high, which can lead to more collisions and potentially more disorder. This high kinetic energy can overcome the tendency for particles to form more stable configurations, affecting the spontaneity of the reaction.
Outlines
🔥 Understanding Enthalpy and Spontaneity
This paragraph discusses the relationship between enthalpy and the spontaneity of reactions under constant pressure. It explains that a negative change in enthalpy (ΔH < 0) indicates an exothermic reaction, releasing energy, while a positive change (ΔH > 0) indicates an endothermic reaction, absorbing energy. The concept of spontaneity is introduced, suggesting that reactions releasing energy might be spontaneous. However, the narrator hints that other factors, such as entropy and temperature, might also play a role in determining spontaneity.
🌡️ Entropy, Temperature, and Reaction Spontaneity
The second paragraph delves deeper into the factors affecting spontaneity, focusing on entropy and temperature. It describes how a reaction with a negative ΔH and an increase in entropy (ΔS > 0) is likely spontaneous, as it leads to a more disordered state. Conversely, a decrease in entropy (ΔS < 0) might make a reaction less spontaneous, especially at high temperatures. The narrator uses analogies of particles colliding to illustrate how kinetic energy can influence reaction direction and spontaneity, suggesting that high temperatures can disrupt the formation of more stable, lower-energy states.
🔧 High Temperature Effects on Reaction Spontaneity
This paragraph explores the impact of high temperatures on reaction spontaneity. It uses the analogy of car collisions to explain how high kinetic energy can prevent particles from forming stable bonds, leading to a preference for the reverse reaction at high temperatures. The narrator suggests that while low temperatures favor the forward reaction due to particles drifting closer together, high temperatures increase the likelihood of particles ricocheting off each other, making the reverse reaction more spontaneous.
📉 Formulating the Gibbs Free Energy Equation
The final paragraph introduces the concept of Gibbs free energy (ΔG) as a predictor of reaction spontaneity. It proposes a formula that combines enthalpy, entropy, and temperature to determine if a reaction is spontaneous. The narrator explains that a negative ΔG indicates a spontaneous reaction, while a positive ΔG suggests the reaction is non-spontaneous. The paragraph concludes by affirming that this intuitive understanding aligns with the actual thermodynamic principles that will be discussed in more detail in future videos.
Mindmap
Keywords
💡Enthalpy
💡Constant Pressure
💡Exothermic Reaction
💡Endothermic Reaction
💡Spontaneity
💡Entropy
💡Second Law of Thermodynamics
💡Gibbs Free Energy
💡Kinetic Energy
💡Temperature
💡Macro and Micro States
Highlights
The change in enthalpy is equal to the heat added to a system at constant pressure.
An exothermic reaction is indicated by a negative change in enthalpy, meaning energy is released.
Endothermic reactions absorb energy, indicated by a positive change in enthalpy.
Spontaneity of a reaction may not be solely determined by enthalpy; entropy and temperature also play crucial roles.
Entropy tends to increase in natural processes, suggesting a move towards more ordered states is less spontaneous.
High temperatures can influence the spontaneity of reactions by affecting the kinetic energy of particles.
A reaction with a negative enthalpy and positive entropy is likely to be spontaneous.
In contrast, a reaction with negative enthalpy but negative entropy may not be spontaneous at high temperatures.
The concept of Gibbs free energy, delta G, is introduced as a predictor of reaction spontaneity.
The formula for spontaneity involves enthalpy, entropy, and temperature, weighted to reflect their relative impacts.
At low temperatures, the enthalpy term dominates in determining spontaneity.
At high temperatures, the entropy term, when scaled by temperature, can override the enthalpy term.
The spontaneity of a reaction can shift with changes in temperature, affecting both the forward and reverse reactions.
The video aims to provide an intuitive understanding of why the formula for spontaneity makes sense.
The formula for Gibbs free energy will be further explored in future videos, connecting it to fundamental thermodynamic principles.
Transcripts
Browse More Related Video

Gibbs free energy and spontaneous reactions | Biology | Khan Academy
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
[H2 Chemistry] 2021 Topic 5 Energetics 3

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry

Endergonic, exergonic, exothermic, and endothermic reactions | Khan Academy

Entropy: Embrace the Chaos! Crash Course Chemistry #20
5.0 / 5 (0 votes)
Thanks for rating: