Endergonic, exergonic, exothermic, and endothermic reactions | Khan Academy
TLDRThe video script discusses the concepts of exothermic and endothermic reactions, as well as exergonic and endergonic processes, relating them to changes in enthalpy, entropy, and Gibbs free energy. It explains how these reactions and processes can be spontaneous or non-spontaneous based on the thermodynamic properties and the conditions such as temperature. The script uses clear examples and analogies to illustrate how energy transformations at the microscopic level affect the spontaneity of reactions.
Takeaways
- 🌡️ Exothermic reactions release heat, characterized by a negative change in enthalpy (ΔH < 0).
- 🔥 The heat released in an exothermic reaction can be viewed as a decrease in heat content from before to after the reaction.
- 🌞 Endothermic reactions absorb heat, with an enthalpy change that is positive (ΔH > 0), meaning higher enthalpy post-reaction.
- 💺 Ergonomic relates to 'ergon,' which means work, leading to the terms exergonic and endergonic.
- ⚡️ Exergonic reactions release work energy, typically associated with a negative change in Gibbs free energy (ΔG < 0), indicating spontaneity.
- 🔄 Endergonic reactions absorb or use work energy, usually linked with a positive change in Gibbs free energy (ΔG > 0), and are non-spontaneous.
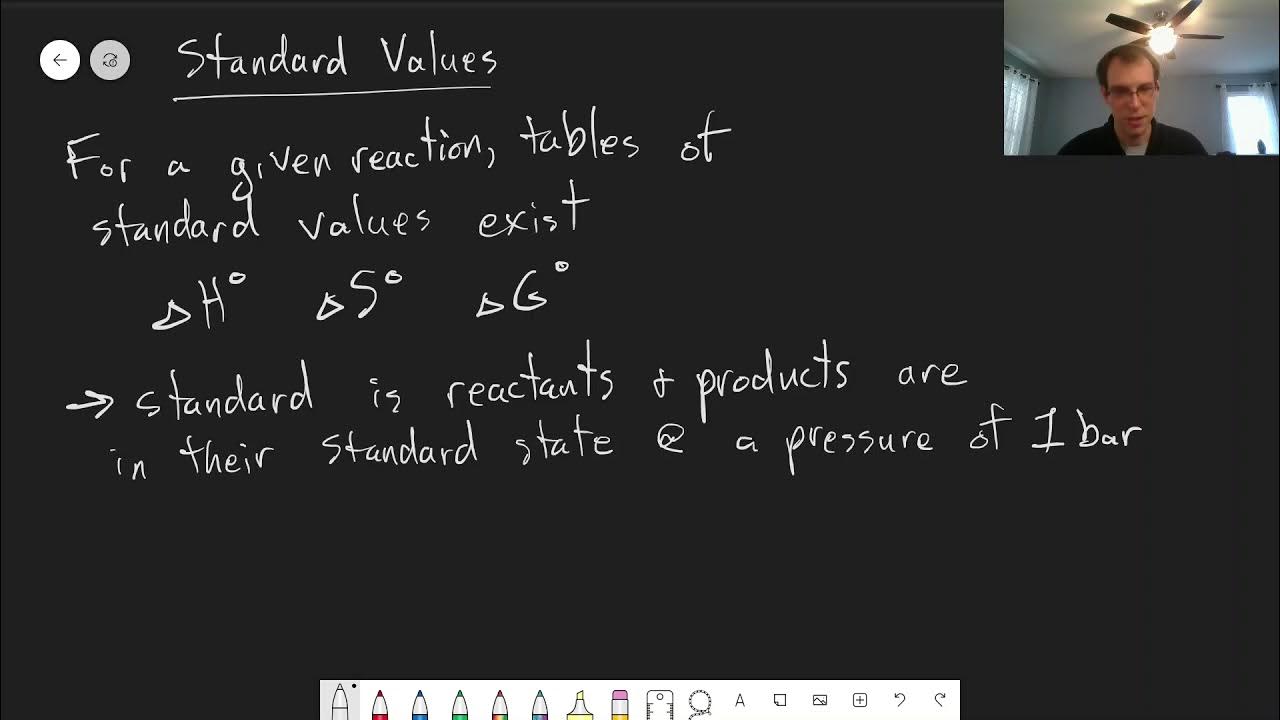
- 📈 Gibbs free energy (G) at constant pressure and temperature is defined as the change in enthalpy minus the product of temperature and change in entropy.
- 🔄 The formula for Gibbs free energy (ΔG) is ΔG = ΔH - TΔS, where T is the temperature and ΔS is the change in entropy.
- 🌟 High temperature can drive endothermic reactions to be spontaneous by increasing kinetic energy and entropy.
- 🔄 In spontaneous reactions, the overall entropy of the system increases, leading to a natural tendency towards a more disordered state.
- 🚫 Non-spontaneous reactions require external energy input to proceed and are less likely to occur without intervention.
Q & A
What is the meaning of the term 'exothermic' in the context of the script?
-Exothermic refers to a reaction that releases heat. It is rooted in the word 'therm', which relates to heat, and indicates a negative change in enthalpy, meaning less heat content after the reaction than before.
How is the enthalpy change (ΔH) related to heat absorption or release in a reaction?
-A negative ΔH indicates that heat is released during the reaction, while a positive ΔH would indicate heat absorption. This is connected to the concept of exothermic and endothermic reactions, respectively.
What does the term 'endothermic' imply about a process?
-Endothermic processes absorb heat, as indicated by the prefix 'endo' and the root 'therm'. It is associated with an increase in enthalpy after the reaction, meaning the system has more heat content after the reaction than before.
How is the concept of 'exergonic' related to work energy?
-Exergonic reactions release work energy. The root 'ergon' comes from Greek for work, implying that in such reactions, work energy is released, often associated with a negative change in Gibbs free energy (ΔG).
What is the definition of 'endergonic' in the context of energy and work?
-Endergonic reactions absorb or use work energy. This term is derived from 'ergon' meaning work, and indicates that the reaction requires work energy to proceed, typically associated with a positive ΔG.
What is the formula for Gibbs free energy at constant pressure and temperature?
-The formula for Gibbs free energy (ΔG) at constant pressure and temperature is ΔG = ΔH - TΔS, where ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy.
How does the script explain the relationship between heat, entropy, and work energy?
-The script explains that heat can be related to the kinetic energy of molecules, while entropy is associated with the disorder or the number of possible states a system can occupy. Work energy is the energy available for useful work, which is related to the difference between heat and entropy in the context of Gibbs free energy.
Why is an exothermic reaction with an increase in entropy considered spontaneous?
-An exothermic reaction with an increase in entropy is spontaneous because the reaction releases heat, leading to a negative ΔH, and the increase in entropy (ΔS > 0) at a high temperature (T > 0) results in a negative ΔG, which drives the reaction forward.
Under what conditions might an endothermic reaction be spontaneous?
-An endothermic reaction, which absorbs heat, might be spontaneous at high temperatures if the increase in entropy is significant enough to result in a negative ΔG, despite the absorption of heat energy.
What factors contribute to a non-spontaneous exothermic reaction?
-A non-spontaneous exothermic reaction can occur if the decrease in enthalpy (ΔH < 0) is not sufficient to overcome a decrease in entropy (ΔS < 0) at a high temperature, resulting in a positive ΔG.
How does the script illustrate the concept of spontaneous reactions?
-The script illustrates spontaneous reactions by discussing how changes in enthalpy, entropy, and temperature interact to determine the spontaneity of a reaction. It provides examples of how exothermic and endothermic reactions can be spontaneous or non-spontaneous based on these factors.
What can be inferred about the importance of entropy in determining the spontaneity of reactions?
-Entropy plays a crucial role in determining the spontaneity of reactions, especially at high temperatures. An increase in entropy (ΔS > 0) generally favors spontaneity, while a decrease in entropy (ΔS < 0) can make a reaction non-spontaneous, unless other factors like a significant release of heat (exothermic) overcome it.
Outlines
🌡️ Understanding Exothermic and Endothermic Reactions
This paragraph introduces the concepts of exothermic and endothermic reactions, highlighting their relation to energy absorption and release. The voiceover explains that exothermic reactions release heat, as indicated by a negative change in enthalpy, while endothermic reactions absorb heat, indicated by a positive change in enthalpy. The paragraph also touches on the concepts of constant pressure and how it relates to these reactions, as well as the biological systems where these processes occur. The discussion then moves to exergonic and endergonic processes, which are related to the release and absorption of work energy, respectively. The paragraph concludes with an introduction to Gibbs free energy and its formula, explaining how it relates to the energy available for work and the spontaneity of reactions.
🔧 Examining Spontaneity in Exothermic and Exergonic Reactions
This paragraph delves into the spontaneity of exothermic and exergonic reactions, providing scenarios to illustrate why they make intuitive sense. It explains that an exothermic reaction with a negative delta H releases heat and increases entropy, leading to a negative delta G, indicating a spontaneous process. The paragraph then discusses a counterintuitive case where an endothermic reaction absorbs heat but can still be spontaneous at high temperatures due to the increase in entropy. The explanation involves the kinetic energy of molecules and the likelihood of reactions proceeding in a particular direction based on the temperature and entropy changes.
🚧 Analyzing Non-Spontaneous Exothermic and Endothermic Reactions
The final paragraph examines scenarios where exothermic and endothermic reactions are not spontaneous. It describes a case where an exothermic reaction reduces entropy and therefore does not occur spontaneously, even though it releases heat. The high temperature and the importance of entropy at such conditions are emphasized. The paragraph then contrasts this with an endothermic reaction that has a reduction in entropy and is not spontaneous, despite the potential for energy release. The discussion concludes with an explanation of why reactions that require heat and result in decreased entropy are unlikely to occur spontaneously, especially in chaotic, high-temperature environments.
Mindmap
Keywords
💡Exothermic
💡Enthalpy
💡Endothermic
💡Ergonic
💡Gibbs Free Energy
💡Entropy
💡Spontaneous Reaction
💡Kinetic Energy
💡Temperature
💡Non-Spontaneous Reaction
💡Molecular Configuration
Highlights
The concept of exothermic reactions is introduced, which are reactions that release heat.
The root of the word exothermic, 'therm', relates to heat, signifying a heat-releasing process.
A negative change in enthalpy indicates heat release, aligning with the concept of exothermic reactions.
Endothermic reactions are defined as processes that absorb heat, contrasting with exothermic reactions.
The prefix 'endo' in endothermic indicates heat absorption, as opposed to 'exothermic' which implies heat release.
Enthalpy as a measure of heat content, where a lower post-reaction enthalpy signifies heat release.
The relationship between exergonic and endergonic reactions and their respective energy work release or absorption.
The term 'ergon' from Greek, meaning 'work', is the root for exergonic, indicating a reaction that releases work energy.
Gibbs free energy is introduced as a variable to consider when thinking about energy for work in reactions.
The formula for Gibbs free energy at constant pressure and temperature is provided, linking enthalpy, entropy, and temperature.
The relationship between heat absorption or release and the change in Gibbs free energy.
Spontaneous reactions are characterized by a delta G less than zero, indicating an exergonic process.
Non-spontaneous reactions have a delta G greater than zero, typically associated with endergonic processes.
An exothermic reaction with an increase in entropy is described as spontaneous due to a negative delta G.
A counterintuitive example of an endothermic reaction that is spontaneous at high temperatures is discussed.
The importance of entropy in high-temperature systems and its role in determining the spontaneity of reactions.
An exothermic reaction can be non-spontaneous if it results in a decrease in entropy at high temperatures.
An endothermic and endergonic reaction that is non-spontaneous due to an increase in entropy and positive delta G.
The concept of chaos and high kinetic energy in systems affecting the direction of reactions and entropy.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: