[H2 Chemistry] 2021 Topic 5 Energetics 3
TLDRThis educational video script delves into the advanced concepts of energetics, focusing on entropy and Gibbs free energy. It introduces entropy change (ΔS) and its role alongside enthalpy change (ΔH) in determining spontaneity of reactions through Gibbs free energy change (ΔG). The script guides students through the relationship between these thermodynamic quantities and temperature, emphasizing the importance of understanding state functions and the impact of temperature on reaction spontaneity. It also touches on the limitations of ΔG in predicting reaction kinetics, highlighting the distinction between thermodynamic feasibility and kinetic barriers.
Takeaways
- 📚 The section covers new material on energetics, focusing on concepts not typically covered in secondary school.
- 🔥 Initial topics include heat transfer and enthalpy change, with a recap on earlier sections about calorimetry.
- 🌡️ Introduction of two new terms: entropy (ΔS) and Gibbs free energy (ΔG), explaining their significance and relationship with enthalpy (ΔH).
- 🔍 Entropy change (ΔS) is related to the spontaneity of processes, illustrated with examples like perfume diffusion and dye mixing in water.
- 💡 Spontaneous processes are those that occur without continuous energy input, with examples of exothermic and endothermic reactions.
- 🔄 Entropy change is influenced by the degree of freedom and distribution of energy, with states like solid, liquid, and gas having different entropies.
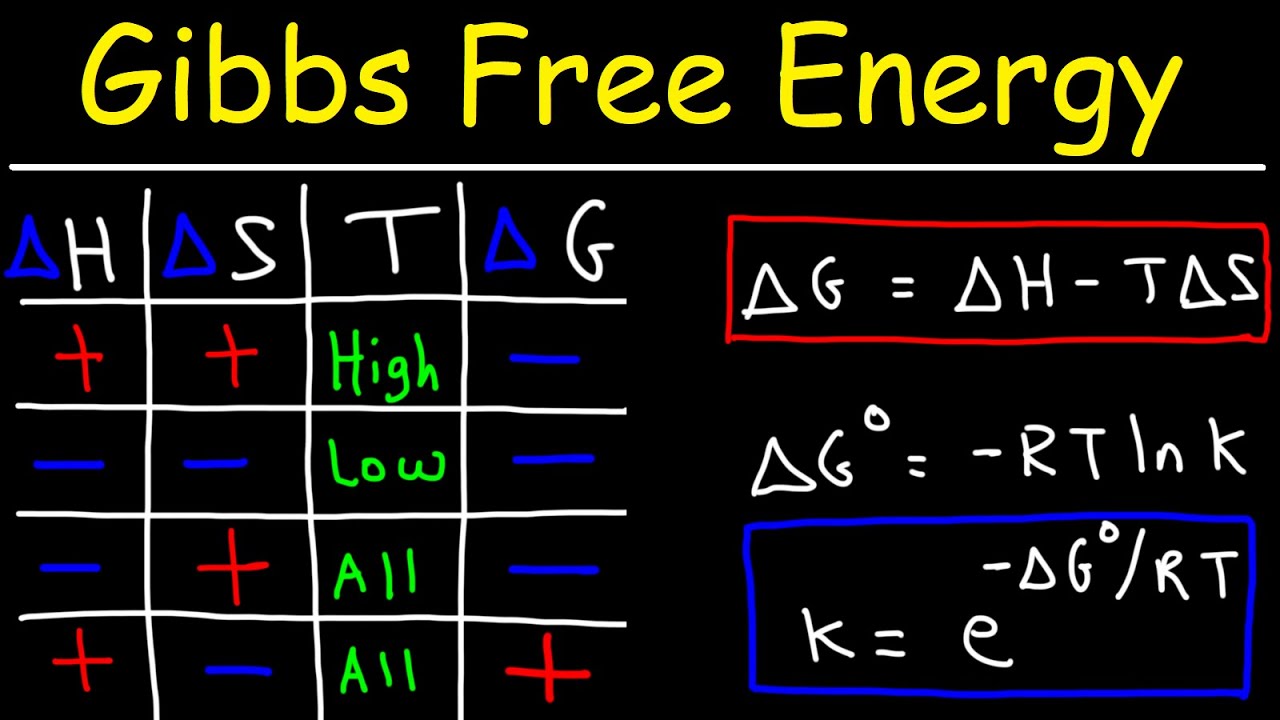
- 📈 Calculation and prediction of spontaneity using Gibbs free energy (ΔG = ΔH - TΔS), emphasizing the importance of sign and units in calculations.
- 🔄 Phase changes (solid to liquid, liquid to gas) typically result in positive entropy changes due to increased molecular freedom.
- 🔬 The relationship between entropy and temperature is discussed, explaining the Maxwell-Boltzmann distribution and energy dispersion.
- 🧮 Exercises and calculations are crucial for understanding and applying the concepts, with an emphasis on avoiding common mistakes, like unit mismatches.
Q & A
What is the main focus of the chapter on energetics?
-The main focus of the chapter on energetics is to introduce and explain concepts such as heat transfer, enthalpy change, entropy, and free energy change, and how these concepts relate to the spontaneity of reactions.
What is a state function in thermodynamics?
-A state function in thermodynamics is a property that depends only on the current state of the system and not on the path taken to reach that state. Examples given in the script include enthalpy (ΔH) and entropy (ΔS).
What is the relationship between ΔG (Gibbs free energy change), ΔH, and ΔS?
-The relationship between Gibbs free energy change (ΔG), enthalpy change (ΔH), and entropy change (ΔS) is given by the equation ΔG = ΔH - TΔS, where T is the temperature in Kelvin.
How does the concept of spontaneity relate to the diffusion of perfume in a room?
-The concept of spontaneity is illustrated by the diffusion of perfume in a room. When perfume is sprayed in one corner of a room, it spontaneously diffuses to the other side, demonstrating a natural process that does not require continuous input of energy.
What is the difference between an exothermic and endothermic reaction in terms of spontaneity?
-Exothermic reactions release energy and are often associated with spontaneity, as in the case of combustion. However, endothermic reactions, which absorb energy, can also be spontaneous under certain conditions, such as when there is an increase in entropy.
Why is the term 'entropic term' introduced in the context of non-spontaneous reactions?
-The entropic term (ΔS) is introduced because it is another important factor, along with enthalpy change (ΔH), that determines the spontaneity of a reaction. It accounts for the changes in the degree of freedom and the dispersal of energy in a system.
What does it mean for a process to have a greater degree of freedom?
-A process has a greater degree of freedom when there are more ways to distribute or disperse energy among the particles in the system, often associated with an increase in entropy.
How does the change in phase from solid to gas affect entropy?
-The change in phase from solid to gas results in an increase in entropy because gaseous particles have more freedom to move and distribute energy compared to solid particles, which are restricted to vibrating about fixed positions.
What is the significance of the Gibbs free energy equation in predicting the spontaneity of a reaction?
-The Gibbs free energy equation (ΔG = ΔH - TΔS) is significant in predicting the spontaneity of a reaction because a negative ΔG indicates that the reaction is spontaneous, while a positive ΔG suggests that the reaction is not spontaneous under standard conditions.
What are the limitations of using ΔG to predict the spontaneity of a reaction?
-The limitations of using ΔG to predict spontaneity include uncertainties under non-standard conditions where ΔH and ΔS may change, and the fact that high activation energy can prevent a spontaneous reaction from occurring even if ΔG is negative, due to kinetic constraints.
Outlines
🔍 Introduction to Advanced Energetics Concepts
The script begins with an overview of the energetics chapter, introducing the audience to more complex topics beyond basic ideas like heat transfer and enthalpy change. The lecturer emphasizes the importance of understanding the relationship between different energetic terms and introduces the concept of calorimetry, which involves measuring temperature changes to determine the limiting reagent and calculate enthalpy change (∆H). The focus then shifts to introducing two new key terms: entropy (∆S) and Gibbs free energy change (∆G), explaining their significance in determining the spontaneity of a process.
🌡️ Understanding Entropy and Spontaneity
This paragraph delves into the concept of entropy, defining it as a state function and discussing its role in spontaneous processes. The lecturer uses the diffusion of perfume and the dispersion of ink in water as examples to illustrate spontaneity. The historical context of thermodynamics is briefly mentioned, and the idea of thermodynamic coupling in non-spontaneous processes is introduced. The paragraph also explains how the degree of freedom in a system, such as the transition from solid to gas, affects entropy and the spontaneity of a process.
📈 Entropy Change and Its Impact on Reaction Spontaneity
The script continues by discussing how entropy change (∆S) is represented and its connection to absolute entropy. It explains that an increase in the degree of freedom, such as in phase changes or the dissolution of solids, leads to a positive entropy change, which is favored for spontaneity. The relationship between entropy, temperature, and the distribution of energy among particles is explored, using the Maxwell-Boltzmann distribution curve as a visual aid to understand how temperature affects entropy.
🌟 Key Factors Influencing Entropy Change
This section highlights the factors that influence entropy change, such as temperature increase, phase change, and the increase in the number of gaseous particles. It emphasizes the importance of understanding these factors when predicting the spontaneity of a reaction. The concept of absolute entropy for different states of matter (solid, liquid, gas) is introduced, and the expectation of positive entropy change during phase transitions, such as solid to liquid or liquid to gas, is explained.
💧 Dissolution, Mixing, and the Complexity of Entropy
The paragraph discusses the entropy changes associated with the dissolution of ionic solids and the mixing of liquids with similar polarities. It points out the complexity of predicting entropy changes during dissolution due to the ordered arrangement of water molecules around ions, which might cause a local decrease in entropy. The讲师 also touches on the concept of Gibbs free energy and its significance in predicting the spontaneity of reactions, using the formula ∆G = ∆H - T∆S.
⚗️ Calculating Gibbs Free Energy and Its Significance
This section focuses on the calculation of Gibbs free energy (∆G) and the importance of units when performing these calculations. The script warns against common mistakes, such as incorrect unit conversions, which can lead to erroneous results. It also introduces exercises for calculating ∆G from ∆H and ∆S and discusses the temperature dependence of ∆G, highlighting the need to consider phase changes and their impact on thermodynamic values.
🔍 The Effect of Temperature on Reaction Spontaneity
The script explores how temperature affects the spontaneity of a reaction by influencing ∆G. It explains that reactions with negative ∆H and positive ∆S are spontaneous at all temperatures, while those with opposite signs are not spontaneous. The paragraph also discusses enthalpy-driven and entropy-driven reactions, and how temperature can influence which type of reaction is spontaneous. The讲师 encourages students to work through examples to understand these concepts better.
🏔️ Limitations of Gibbs Free Energy in Predicting Spontaneity
The final paragraph addresses the limitations of using Gibbs free energy to predict the spontaneity of reactions, particularly under non-standard conditions where ∆H and ∆S may not be accurately known. It also introduces the concept of kinetics considerations, explaining that even spontaneous reactions may not occur if the activation energy is too high. The script concludes by emphasizing the importance of understanding both thermodynamics and kinetics when predicting the likelihood of a reaction.
Mindmap
Keywords
💡Enthalpy Change
💡Entropy
💡Free Energy Change
💡Spontaneity
💡Thermodynamic Coupling
💡Gibbs Free Energy
💡Calorimetry
💡Limiting Reagent
💡Degree of Freedom
💡Phase Change
💡Temperature Dependence
Highlights
Introduction to section 8, the last part of the energetics chapter, covering new concepts such as entropy and free energy change.
Explanation of the relationship between different energetics topic terms via the hair cycle.
Introduction to calorimetry and its practical application in measuring temperature changes for determining limiting reagents and enthalpy change.
Focus on the importance of entropy (ΔS) and its role alongside enthalpy change (ΔH) in determining spontaneity of reactions.
The concept of Gibbs free energy change (ΔG) and its relation to ΔH and ΔS with the formula ΔG = ΔH - TΔS.
Understanding spontaneity through examples such as perfume diffusion and ink dispersion in water.
The significance of the entropic component in reactions and its contribution to the overall Gibbs free energy change.
Historical context of thermodynamics and the importance of studying the work of scientists who developed these concepts.
Explanation of thermodynamic coupling and its role in non-spontaneous processes coupled with spontaneous reactions.
The concept of degree of freedom and its impact on the direction of spontaneity and entropy change.
How the phase change of substances affects entropy, with solids having less entropy than liquids and gases.
The impact of temperature change on entropy, demonstrating that increasing temperature leads to positive entropy change.
The relationship between the number of moles of gaseous particles and entropy change, with an increase leading to a positive change.
Illustration of entropy using the Maxwell-Boltzmann distribution curve and its broadening at higher temperatures.
Discussion on the dissolution of ionic solids and the balance between increased freedom of ions and the ordered fashion of water molecules around them.
The Gibbs free energy expression and its significance in predicting the spontaneity of reactions at standard conditions.
The limitations of using ΔG to predict spontaneity, including non-standard conditions and kinetic considerations.
Emphasis on the importance of understanding both thermodynamics and kinetics for a comprehensive study of chemical reactions.
Transcripts
Browse More Related Video

Gibbs free energy and spontaneous reactions | Biology | Khan Academy

Gibbs free energy and spontaneity | Chemistry | Khan Academy
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
Chapter 6: K and Standard Gibbs Energy | CHM 214 | 051

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry

Gibbs free energy example | Thermodynamics | Chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: