Gibbs free energy and spontaneous reactions | Biology | Khan Academy
TLDRThe video script delves into Gibbs Free Energy, a crucial concept in chemistry and biology for predicting the spontaneity of reactions. It introduces the formula for Gibbs Free Energy (ΔG = ΔH - TΔS) and explains how a negative ΔG indicates a spontaneous reaction. The script explores various scenarios based on changes in enthalpy (ΔH) and entropy (ΔS), demonstrating how temperature affects spontaneity. It emphasizes the practicality of this concept in understanding chemical reactions and biological processes, assuming constant pressure and temperature.
Takeaways
- 📚 Gibbs Free Energy is a concept used to predict the spontaneity of chemical and biological reactions.
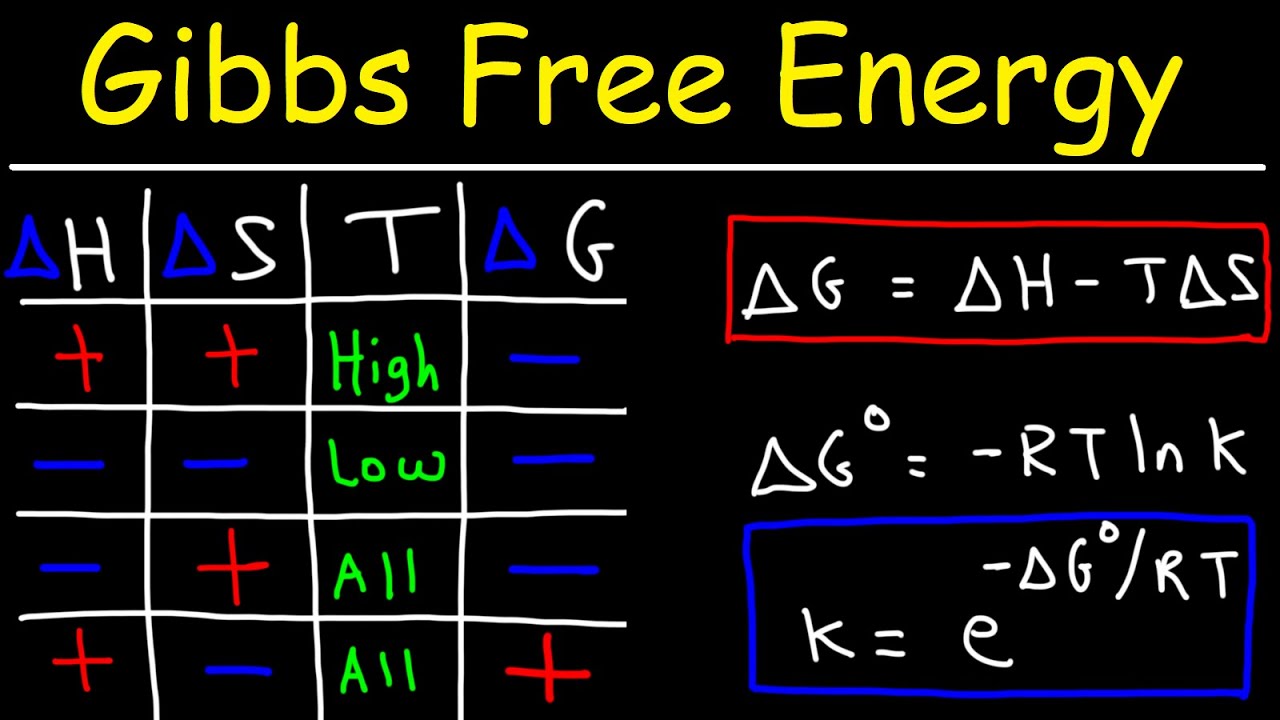
- 🔍 It was defined by Josiah Willard Gibbs and is calculated using the formula ΔG = ΔH - TΔS, where ΔH is the change in enthalpy, T is the temperature, and ΔS is the change in entropy.
- 🌡️ The formula applies under conditions of constant pressure and temperature, which are common in many chemical and biological systems.
- 🚀 A reaction is spontaneous if ΔG is less than zero, meaning it will occur without external intervention.
- 🔄 If ΔH is negative and ΔS is positive, the reaction is spontaneous at all temperatures due to the release of energy and increase in disorder.
- ⚠️ A reaction with ΔH greater than zero and ΔS less than zero is non-spontaneous because it requires energy and results in a decrease in disorder.
- 🌞 At low temperatures, reactions with ΔH negative and ΔS negative can be spontaneous if the release of energy outweighs the decrease in entropy.
- 🔥 At high temperatures, reactions with ΔH positive and ΔS positive can be spontaneous because the increase in disorder can outweigh the energy requirement.
- 🌐 The Second Law of Thermodynamics is not violated as the total entropy of the universe increases due to heat released during spontaneous reactions.
- 🔍 Gibbs Free Energy is a useful tool for understanding and predicting the natural tendencies of chemical and biological processes.
- 📈 In practice, ΔG values help identify whether a reaction is spontaneous, at equilibrium, or non-spontaneous.
Q & A
What is Gibbs Free Energy?
-Gibbs Free Energy is a thermodynamic potential that measures the maximum reversible work that can be done by a system at constant temperature and pressure.
Who defined Gibbs Free Energy?
-Gibbs Free Energy was defined by Josiah Willard Gibbs, an American scientist.
How is the change in Gibbs Free Energy calculated?
-The change in Gibbs Free Energy (ΔG) is calculated using the formula ΔG = ΔH - TΔS, where ΔH is the change in enthalpy, T is the temperature, and ΔS is the change in entropy.
What does a negative ΔG value indicate?
-A negative ΔG value indicates that a reaction is spontaneous, meaning it will occur naturally without the need for external energy input.
What does a positive ΔG value signify?
-A positive ΔG value signifies that a reaction is non-spontaneous under the given conditions. It requires energy input to proceed.
How does enthalpy (ΔH) relate to the spontaneity of a reaction?
-If ΔH is negative (exothermic reaction), the system releases energy, which can lead to a spontaneous reaction. If ΔH is positive (endothermic reaction), the system absorbs energy, which generally makes the reaction non-spontaneous.
What role does entropy (ΔS) play in determining spontaneity?
-Entropy is a measure of disorder. An increase in entropy (ΔS > 0) can contribute to the spontaneity of a reaction, especially at higher temperatures, by making the reaction more likely to occur.
How does temperature affect the spontaneity of a reaction?
-Temperature affects the spontaneity by influencing the TΔS term in the Gibbs Free Energy equation. At low temperatures, enthalpy changes are more critical, while at high temperatures, entropy changes can drive spontaneity.
What happens when a reaction has both ΔH and ΔS values less than zero?
-If both ΔH and ΔS are less than zero, the reaction is definitely spontaneous because the negative ΔG resulting from the equation will be further decreased by the negative TΔS term.
Can a reaction with a positive ΔH and a positive ΔS be spontaneous?
-Yes, a reaction with a positive ΔH and a positive ΔS can be spontaneous at high temperatures. The TΔS term can become large enough to overcome the positive ΔH, resulting in a negative ΔG.
What is the significance of Gibbs Free Energy in chemistry and biology?
-Gibbs Free Energy is crucial in predicting the spontaneity of reactions in chemical and biological systems, helping to determine whether a process will occur naturally or if it requires energy input.
Outlines
🌟 Introduction to Gibbs Free Energy
This paragraph introduces the concept of Gibbs Free Energy, emphasizing its significance in determining the spontaneity of chemical and biological reactions. It explains that Gibbs Free Energy, defined by Josiah Willard Gibbs, is calculated using a formula involving changes in enthalpy (heat content) and entropy (disorder) at constant pressure and temperature. The paragraph highlights that a negative change in Gibbs Free Energy (ΔG < 0) indicates a spontaneous reaction, while a positive change (ΔG > 0) suggests a non-spontaneous one. It further discusses scenarios where the spontaneity of a reaction depends on the temperature and the interplay between enthalpy and entropy changes, providing a foundational understanding of the principles behind Gibbs Free Energy.
🔍 Analyzing Spontaneity with Gibbs Free Energy
The second paragraph delves deeper into the analysis of reaction spontaneity using Gibbs Free Energy. It explores the impact of temperature on the spontaneity of reactions, explaining how low temperatures may favor reactions that release energy and decrease entropy, while high temperatures can make reactions that increase entropy spontaneous despite an increase in enthalpy. The paragraph also clarifies that even reactions with a decrease in entropy can be spontaneous if they release heat, which adds to the overall entropy of the universe, thus aligning with the Second Law of Thermodynamics. The summary underscores the importance of Gibbs Free Energy in predicting the spontaneity of reactions under various conditions and its applicability to chemical and biological systems.
Mindmap
Keywords
💡Gibbs Free Energy
💡Spontaneity
💡Enthalpy
💡Entropy
💡Temperature
💡Chemical Reactions
💡Biological Systems
💡Josiah Willard Gibbs
💡Constant Pressure and Temperature
💡Equilibrium
💡Second Law of Thermodynamics
Highlights
Exploration of Gibbs Free Energy and its role in determining the spontaneity of reactions in chemistry and biology.
The definition of Gibbs Free Energy by Josiah Willard Gibbs and its famous formula for predicting spontaneity.
The formula for change in Gibbs Free Energy, Delta G = Delta H - T * Delta S, where 'H' represents enthalpy and 'S' represents entropy.
The condition for a spontaneous reaction: if Delta G is less than zero.
A reaction releasing energy (negative Delta H) and increasing entropy (positive Delta S) will always be spontaneous, regardless of temperature.
In the case of positive Delta H and negative Delta S, the reaction is non-spontaneous as it leads to an increase in Gibbs Free Energy.
The impact of temperature on reactions with negative Delta H but positive Delta S, where low temperatures favor spontaneity.
How the Second Law of Thermodynamics is maintained even when local entropy decreases, through the release of heat that increases the entropy of the universe.
The scenario where high temperatures can prevent spontaneity due to molecules not interacting properly despite negative Delta H.
The explanation of why certain reactions do not occur spontaneously even if they would increase entropy, due to positive Delta H and low temperatures.
The possibility of spontaneous reactions with positive Delta H at high temperatures due to the overwhelming increase in entropy.
The practical applications of Gibbs Free Energy in understanding and predicting the spontaneity of reactions in test tubes and biological systems.
The importance of constant pressure and temperature assumptions in the use of Gibbs Free Energy formula.
The use of Delta G to identify spontaneous reactions (negative Delta G), equilibrium (zero Delta G), and non-spontaneous processes (positive Delta G).
Transcripts
Browse More Related Video

Gibbs free energy and spontaneity | Chemistry | Khan Academy
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
[H2 Chemistry] 2021 Topic 5 Energetics 3

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry

Chapter 6: Gibbs Free Energy | CHM 214 | 050
Chapter 6: K and Standard Gibbs Energy | CHM 214 | 051
5.0 / 5 (0 votes)
Thanks for rating: