18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry
TLDRIn this chemistry lesson by Chad's Prep, Chad explains the concept of Gibbs free energy (ΔG), which determines the spontaneity of a reaction. He clarifies that ΔG is not about 'free' as in cost-free, but about energy available to do work. Chad derives the equation ΔG = ΔH - TΔS from the second law of thermodynamics, emphasizing its importance in predicting whether a reaction is spontaneous, non-spontaneous, or at equilibrium. He also discusses the relationship between ΔG, ΔH, and ΔS, and how the universe favors exothermic reactions and increased entropy. The lesson includes practical examples, such as phase changes, to illustrate the application of these principles.
Takeaways
- 📚 The lesson is focused on Gibbs free energy, a fundamental concept in thermodynamics, taught by Chad from Chad's Prep.
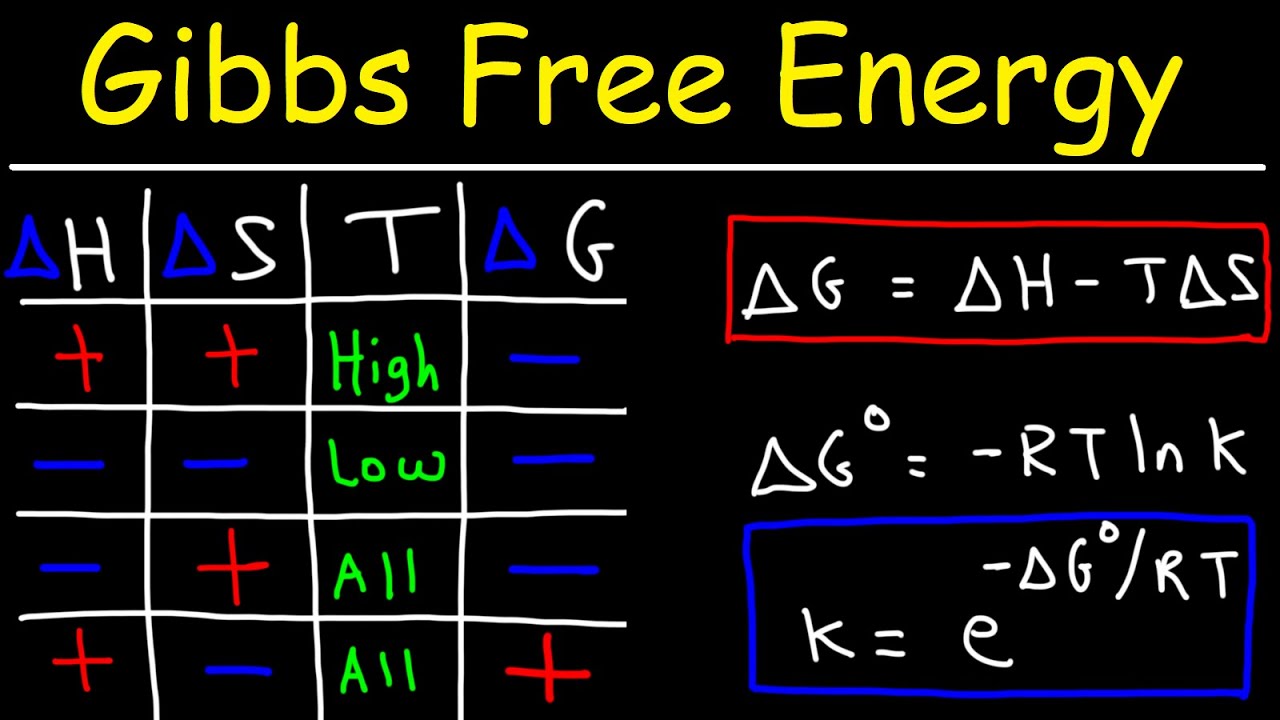
- 🔍 Gibbs free energy (ΔG) is represented by the equation ΔG = ΔH - TΔS, which defines the energy available to do work in a system.
- 🆓 The term 'free' in Gibbs free energy refers to energy that is available or 'free' to do work, not energy that is cost-free.
- 🔄 A negative ΔG indicates a spontaneous reaction, where the system uses its free energy to accomplish work.
- ⚠️ A positive ΔG signifies a non-spontaneous process, meaning the system cannot proceed without input from the surroundings.
- 🔄 At ΔG equals zero, the system is at equilibrium, where there is no net change in the reaction.
- 🌡 The concept of ΔG standard (ΔG°) is introduced, which refers to the Gibbs free energy under standard conditions, typically at 298 Kelvin.
- 🔢 The mathematical derivation of ΔG from the second law of thermodynamics is highlighted, emphasizing the relationship between ΔS, ΔH, and T.
- ♨️ The universe tends to favor reactions that are exothermic (negative ΔH) and have an increase in entropy (positive ΔS), both of which contribute to a negative ΔG.
- 🔄 The spontaneity of a reaction is conditional upon whether ΔH and ΔS are both positive, both negative, or one of each, affecting whether the reaction is spontaneous at all, no, high, or low temperatures.
- 📉 Chad provides a method to calculate the boiling point of water using the given ΔH and ΔS values, demonstrating the practical application of Gibbs free energy principles.
Q & A
What is the primary goal of Chad's Prep?
-The primary goal of Chad's Prep is to take the stress out of learning science, offering high school and college science prep, as well as MCAT, DAT, and OAT prep.
What does the symbol 'ΔG' represent in thermodynamics?
-'ΔG' represents the change in Gibbs free energy, which is a measure of the energy available to do work in a system.
How is the change in Gibbs free energy, 'ΔG', mathematically defined in terms of other thermodynamic quantities?
-'ΔG' is mathematically defined as ΔH minus T ΔS, where ΔH is the change in enthalpy, T is the temperature, and ΔS is the change in entropy.
What is the physical interpretation of a negative 'ΔG' value?
-A negative 'ΔG' value indicates that a reaction is spontaneous, meaning the system has enough free energy to accomplish the reaction.
What does a positive 'ΔG' signify in the context of a chemical reaction?
-A positive 'ΔG' signifies a non-spontaneous process, where the system cannot proceed with the reaction without input of energy from the surroundings.
What is the significance of 'ΔG' being equal to zero?
-When 'ΔG' equals zero, the system is at equilibrium, meaning the forward and reverse reactions are occurring at the same rate.
What is the difference between 'ΔG' and 'ΔG°' (Delta G standard)?
-'ΔG°', or Delta G standard, refers to the change in Gibbs free energy under standard conditions, typically at 298 Kelvin and with specific concentrations or pressures for reactants and products.
How does the second law of thermodynamics relate to the spontaneity of a reaction?
-The second law of thermodynamics states that the total entropy change of the universe (system + surroundings) must be positive for a process to be spontaneous.
What are the two conditions that the universe favors for a spontaneous reaction according to the script?
-The universe favors exothermic reactions (negative ΔH) and reactions with an increase in entropy (positive ΔS) for the system.
How can you determine the threshold temperature for a reaction to be spontaneous?
-The threshold temperature can be determined by setting 'ΔG' to zero and solving for the temperature T in the equation ΔG = ΔH - TΔS, using the given values for ΔH and ΔS.
What is the relationship between the boiling point of a substance and 'ΔG'?
-At the boiling point, the substance is in equilibrium between liquid and gas phases, which means 'ΔG' equals zero. This allows for the calculation of ΔS for the vaporization process using the equation ΔS = ΔHvap/Tboil.
Why is it important to match units when calculating 'ΔG' or other thermodynamic quantities?
-Matching units is crucial for accurate calculations. For instance, both ΔH and ΔS should be in the same unit, either joules or kilojoules, to ensure the correct result when determining spontaneity or equilibrium temperatures.
Outlines
🔬 Introduction to Gibbs Free Energy
Chad introduces the concept of Gibbs free energy in the context of his science education platform, Chad's Prep. He explains that Gibbs free energy (ΔG) is a thermodynamic potential used to determine the spontaneity of a process. The formula ΔG = ΔH - TΔS is presented, where ΔH is the change in enthalpy, T is the temperature, and ΔS is the change in entropy. Chad emphasizes that a negative ΔG indicates a spontaneous reaction, a positive ΔG indicates a non-spontaneous reaction, and ΔG equals zero at equilibrium. The distinction between standard Gibbs free energy (ΔG°) and non-standard conditions is also introduced, with a clarification that standard conditions typically refer to concentrations of reactants and products, not necessarily the temperature.
📚 Derivation and Significance of Gibbs Free Energy
Chad discusses the derivation of Gibbs free energy, starting from the second law of thermodynamics which states that the total entropy change of the universe must be positive for a process to be spontaneous. He explains the mathematical transformation that leads to the formula ΔG = ΔH - TΔS, and highlights its significance in determining the spontaneity of a reaction without needing to measure the entropy change of the surroundings. Chad also underscores the importance of understanding this equation for students preparing for exams and further studies in thermodynamics.
🌐 The Universe's Preferences in Chemical Reactions
This paragraph delves into the preferences of the universe regarding chemical reactions, focusing on the tendencies for spontaneous processes. Chad explains that the universe favors exothermic reactions (negative ΔH) and positive entropy changes (positive ΔS). He outlines scenarios where both, one, or neither of these conditions are met, and how they relate to the spontaneity of a reaction. Chad also introduces the concept that the universe tends toward lower energy states and increased disorder, which aligns with the principles of entropy and enthalpy changes.
🔢 Mathematical Relationships Between ΔG, ΔH, and ΔS
Chad provides a mathematical perspective on the relationship between Gibbs free energy, enthalpy change, and entropy change. He discusses the outcomes of adding positive and negative numbers and how these principles apply to the formula ΔG = ΔH - TΔS. The explanation includes how the signs of ΔH and ΔS influence the spontaneity of a reaction, with a focus on the conditions that lead to a negative, positive, or zero ΔG. Chad also emphasizes the importance of understanding these mathematical relationships for predicting reaction spontaneity.
🌡️ Temperature's Role in Reaction Spontaneity
The role of temperature in determining the spontaneity of reactions is explored in this paragraph. Chad explains how different combinations of ΔH and ΔS values result in reactions being spontaneous at all temperatures, no temperatures, high temperatures, or low temperatures. He provides examples, such as the boiling of water and melting of ice, to illustrate the concept of threshold temperatures where ΔG equals zero. Chad also discusses the importance of understanding these temperature-dependent conditions for predicting and analyzing chemical reactions.
📉 Calculating Threshold Temperatures for Spontaneity
Chad demonstrates how to calculate the threshold temperature at which a reaction becomes spontaneous by setting ΔG to zero and solving for the temperature using the values of ΔH and ΔS. He uses the example of water's vaporization to show the calculation process and emphasizes the importance of unit consistency. Chad also points out common mistakes students make, such as not converting units correctly or misinterpreting the resulting temperature in Celsius instead of Kelvin.
📚 Conclusion and Study Resources
In the concluding paragraph, Chad summarizes the importance of understanding Gibbs free energy and its relation to reaction spontaneity. He encourages students to practice and master these concepts, offering a general chemistry master course with over 1200 practice questions for further study. Chad also invites students to support the channel through likes and comments and wishes them happy studying as they continue their educational journey.
Mindmap
Keywords
💡Gibbs free energy
💡Spontaneity
💡Enthalpy change (ΔH)
💡Entropy change (ΔS)
💡Second law of thermodynamics
💡System and surroundings
💡Equilibrium
💡Standard conditions
💡Phase changes
💡Threshold temperature
💡Exothermic and endothermic reactions
Highlights
Gibbs free energy (ΔG) is introduced as a crucial concept in thermodynamics, representing the energy available to do work.
ΔG is mathematically defined as ΔG = ΔH - TΔS, linking enthalpy change, temperature, and entropy change.
The concept of 'free' in Gibbs free energy refers to availability for work, not costlessness.
A negative ΔG indicates a spontaneous reaction, while a positive ΔG signifies a non-spontaneous process.
At ΔG equals zero, the system is at equilibrium, with no net change in free energy.
ΔG standard (ΔG°) is the Gibbs free energy under standard conditions, typically at 298 Kelvin.
The derivation of ΔG from the second law of thermodynamics is explained, emphasizing the importance of ΔS and ΔH.
Gibbs' approach to determining spontaneity without measuring the surroundings' ΔS is outlined.
The universe's preference for processes that lower free energy and increase entropy is discussed.
Exothermic reactions (negative ΔH) and positive ΔS are shown to generally favor spontaneity.
A chart is presented to determine spontaneity based on the signs of ΔH and ΔS, and temperature conditions.
The relationship between phase changes, such as boiling or melting points, and ΔG being zero is explained.
An example calculation for the boiling point of water using ΔH and ΔS is demonstrated.
The importance of unit consistency when performing calculations with ΔH and ΔS is emphasized.
A general strategy for students to approach problems involving ΔG, ΔH, and ΔS is suggested, including memorization or mathematical work.
An example problem involving the spontaneity of the reaction N2 + 3H2 to 2NH3 is solved using the provided ΔH and predicted ΔS.
The video concludes with a reminder of the importance of understanding when a reaction is spontaneous based on ΔH, ΔS, and temperature.
Transcripts
Browse More Related Video
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K

Gibbs free energy and spontaneous reactions | Biology | Khan Academy

Gibbs free energy and spontaneity | Chemistry | Khan Academy
[H2 Chemistry] 2021 Topic 5 Energetics 3

Entropy: Embrace the Chaos! Crash Course Chemistry #20
Chapter 6: K and Standard Gibbs Energy | CHM 214 | 051
5.0 / 5 (0 votes)
Thanks for rating: