Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K
TLDRThe video script delves into the concept of Gibbs free energy and its role in determining the spontaneity of chemical reactions. It explains that a negative change in free energy (ΔG) indicates a spontaneous process, while a positive ΔG suggests a non-spontaneous process requiring energy input. The script also covers the relationship between ΔG, enthalpy change (ΔH), and entropy change (ΔS), and how these values can be used to calculate ΔG at 25 degrees Celsius using the formula ΔG = ΔH - TΔS. The process of converting units from joules to kilojoules is highlighted, and examples are provided to illustrate the calculation of ΔG for different reactions. The script further discusses how the maximum work obtainable from a spontaneous process is equivalent to ΔG and the minimum work needed to drive a non-spontaneous process forward is also equal to ΔG. It concludes with an exploration of how changes in ΔG with varying reaction conditions can predict whether a reaction will proceed spontaneously, reach equilibrium, or require additional energy to proceed.
Takeaways
- 🔍 The change in free energy (ΔG) being less than zero indicates a spontaneous process, while ΔG greater than zero signifies a non-spontaneous process.
- ⚖️ At equilibrium, the change in free energy (ΔG) is equal to zero, which corresponds to a reversible process.
- 🔄 The maximum work obtainable from a spontaneous process is equal to the change in free energy (ΔG).
- ♨️ The minimum work required to drive a non-spontaneous process is also equal to ΔG, as additional energy is needed to overcome energy losses like friction.
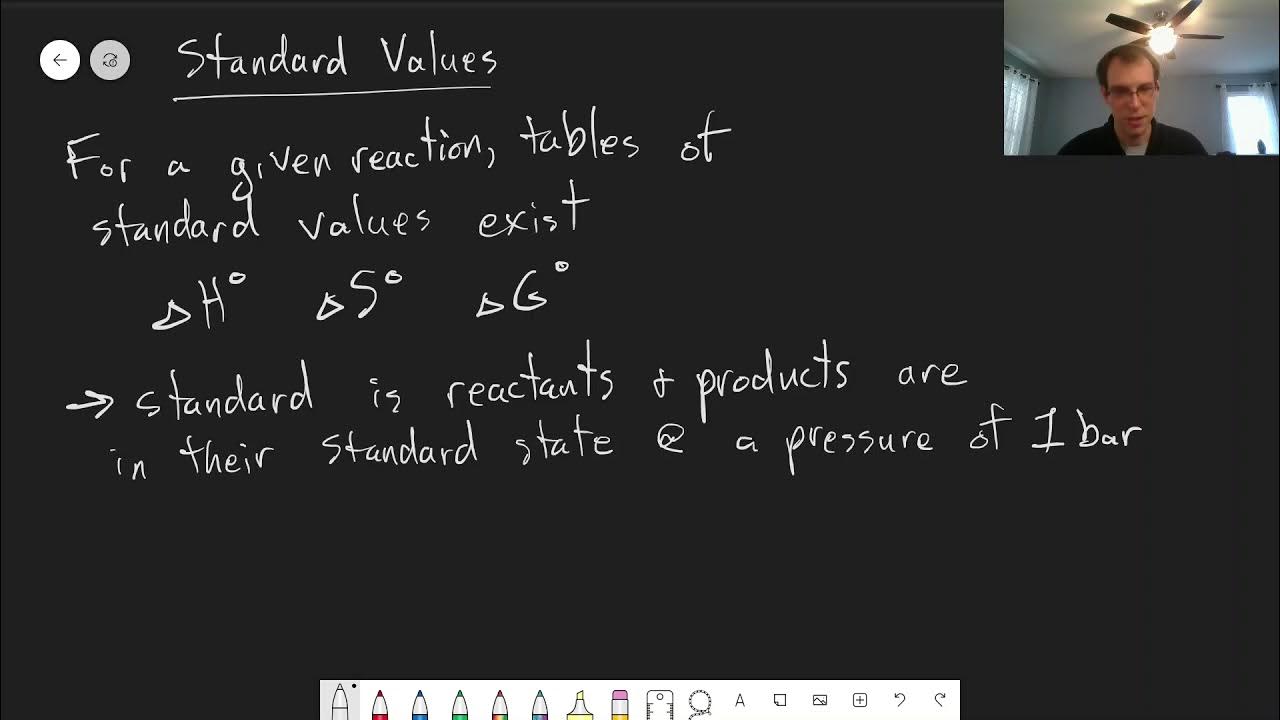
- 📐 Gibbs free energy (ΔG) is calculated using the formula ΔG = ΔH - TΔS, where ΔH is the enthalpy change, T is the temperature, and ΔS is the entropy change.
- ⚙️ Units must be consistent when calculating ΔG, with ΔH and ΔG in kilojoules per mole, and entropy change (ΔS) converted from joules to kilojoules per mole per kelvin.
- 🔢 A negative ΔG value signifies a spontaneous process, while a positive ΔG indicates a non-spontaneous process at a given temperature.
- 🔥 The boiling point of a substance can be calculated using the enthalpy of vaporization and the entropy change associated with the phase transition from liquid to gas.
- 🔀 The equilibrium constant (K) for a reaction is related to the standard free energy change (ΔG°) through the equation ΔG° = -RT ln(K), where R is the gas constant and T is the temperature in Kelvin.
- 🔮 The spontaneity of a reaction and its direction (towards products or reactants) are determined by the signs of ΔH and ΔS, along with the temperature of the system.
- 🧪 For reactions involving the formation of a more ordered state from a less ordered one, such as freezing of water, the process is spontaneous at low temperatures due to the release of energy (exothermic process) and decrease in entropy.
Q & A
What is the significance of the change in free energy (delta G) being less than zero?
-When the change in free energy (delta G) is less than zero, it indicates that a process is spontaneous, meaning it will occur naturally to reach the lowest possible energy state without the input of external energy.
At what value of delta G does a process reach equilibrium?
-A process reaches equilibrium when the change in free energy (delta G) is equal to zero. At this point, the forward and reverse reactions occur at the same rate, and there is no net change in the system.
What is the relationship between the maximum work obtained from a spontaneous process and the change in free energy?
-The maximum amount of work that can be obtained from a spontaneous process is equal to the change in free energy (delta G). This is because the energy released during a spontaneous process can be harnessed to perform work.
How is the change in Gibbs free energy (delta G) calculated at a specific temperature?
-The change in Gibbs free energy (delta G) at a specific temperature is calculated using the formula delta G = delta H - T * delta S, where delta H is the change in enthalpy, T is the temperature in Kelvin, and delta S is the change in entropy.
Why is it necessary to convert the units of entropy from joules to kilojoules when calculating delta G?
-The conversion is necessary to maintain unit consistency, as the change in enthalpy (delta H) and the change in free energy (delta G) are expressed in kilojoules per mole (kJ/mol), while the entropy change is typically given in joules per mole per Kelvin (J/mol·K). Dividing by 1000 converts the entropy change to the same order of magnitude as delta H and delta G.
What does it imply if delta G for a reaction is positive?
-If delta G for a reaction is positive, it implies that the reaction is non-spontaneous under the given conditions. This means that the reaction will not occur naturally without the input of external energy.
How does the entropy change (delta S) affect the spontaneity of a reaction?
-The entropy change (delta S) affects the spontaneity of a reaction through its role in the Gibbs free energy equation. An increase in entropy (positive delta S) can drive a reaction to be spontaneous, even if the enthalpy change (delta H) is positive, depending on the temperature.
What is the boiling point of bromine, and how is it calculated?
-The boiling point of bromine is approximately 59 degrees Celsius. It is calculated using the enthalpy of vaporization and the entropy change of the process. At the boiling point, the Gibbs free energy (delta G) is zero, and the boiling point temperature (T) can be found by rearranging the Gibbs free energy equation to solve for T: T = delta H / delta S.
What is the significance of the equilibrium constant (K) in determining whether a reaction is spontaneous?
-The equilibrium constant (K) is a measure of the ratio of products to reactants at equilibrium. If K is greater than 1, the reaction is product-favored and spontaneous. If K is less than 1, the reaction is reactant-favored and non-spontaneous. If K equals 1, the reaction is at equilibrium with equal concentrations of products and reactants.
How can you determine the standard free energy change (delta G) for a reaction using the equilibrium constant (K)?
-The standard free energy change (delta G) for a reaction can be determined from the equilibrium constant (K) using the equation delta G = -RT ln(K), where R is the gas constant and T is the temperature in Kelvin. This equation allows us to calculate the free energy change when the equilibrium constant is known.
What is the effect of temperature on the spontaneity of the reaction where liquid water turns into ice?
-The reaction where liquid water turns into ice is spontaneous at low temperatures. This is because the process is exothermic (releases heat, delta H is negative) and results in a decrease in entropy (delta S is negative). According to the Gibbs free energy equation, delta G is negative at low temperatures, making the process spontaneous.
Outlines
🔍 Understanding Gibbs Free Energy and Spontaneity
The video begins by addressing the concept of Gibbs free energy and its relation to spontaneous processes. It poses questions about the sign of delta G (change in free energy) in different scenarios: less than zero for spontaneous processes, equal to zero at equilibrium, and greater than zero for non-spontaneous processes. The video clarifies that a negative delta G indicates a spontaneous process, zero at equilibrium, and positive for non-spontaneous processes. It also touches on the relationship between energy, entropy, and the tendency of natural processes to reach the lowest energy state. The key takeaway is that the sign of delta G determines the spontaneity and direction of a chemical reaction.
📐 Calculating Gibbs Free Energy at 25°C
The video continues with a problem-solving approach, guiding viewers on how to calculate the change in Gibbs free energy (delta G) at 25 degrees Celsius. It introduces the formula delta G = delta H - T * delta S, where delta H is the change in enthalpy, T is the temperature in Kelvin, and delta S is the change in entropy. The video emphasizes unit conversion from joules to kilojoules and provides a step-by-step calculation that results in a negative delta G, indicating a spontaneous process. It also explains how to calculate delta G for a reaction using standard enthalpy and entropy values, leading to the conclusion that a negative delta G corresponds to a spontaneous reaction.
🔧 Using Standard Free Energy Change for Reaction Calculations
The video moves on to demonstrate how to calculate the free energy change of a reaction using standard free energy change of formation values. It outlines the process of calculating delta G by multiplying the moles of products and reactants by their respective standard free energy values and subtracting them. The example provided results in a negative delta G, confirming the spontaneity of the reaction. The video also cautions viewers to be careful with the coefficients and signs when performing such calculations.
🔗 Thermochemical Equations and Energy Changes
The discussion shifts to thermochemical equations, explaining how the energy change associated with a reaction is typically expressed in kilojoules. It covers how multiplying coefficients in a balanced equation affects the total energy change and the importance of using kilojoules per mole when calculating non-standard delta G values. The video stresses the need for consistency in units when using the equation delta G = delta G° + RT ln Q, where Q is the reaction quotient, R is the gas constant, and T is the temperature in Kelvin.
🔄 Combining Reactions to Calculate Free Energy Change
The video tackles the calculation of free energy change for the decomposition of dinitrogen pentoxide into dinitrogen tetroxide and oxygen. It shows how to manipulate and combine two given equations to derive the target equation and then sum their adjusted free energy changes to find the overall delta G. The process involves reversing one equation and adjusting coefficients to match the target equation, followed by calculating the new free energy changes and adding them up to get the final result.
🔼 Shift in Free Energy and Reaction Direction
The video explains the implications of a negative standard free energy change on the direction of a reaction and its shift towards equilibrium. It states that a negative delta G indicates a spontaneous reaction that proceeds in the forward direction until equilibrium is reached, where delta G becomes zero. The video also discusses how the free energy change increases as the reaction progresses towards equilibrium from a negative value.
🌡 Estimating the Boiling Point of Bromine
The video provides a method to estimate the boiling point of bromine using the enthalpy of vaporization and standard entropy values for the liquid and gaseous states. It demonstrates the calculation using the equation delta G = delta H - T * delta S, where at the boiling point, delta G is zero, and the system is in equilibrium. The calculation results in the boiling point temperature of bromine, which is approximately 59 degrees Celsius.
⚖️ Evaluating Spontaneity and Equilibrium Constants
The video discusses the relationship between delta G, reaction spontaneity, and the equilibrium constant (K). It explains that a negative delta G corresponds to a spontaneous reaction with K > 1, a positive delta G to a non-spontaneous reaction with K < 1, and a delta G of zero indicates equilibrium with K = 1. The video also addresses the concept that a large K value indicates a product-favored reaction at equilibrium.
🔄 Calculating Standard Free Energy Change from Kp
The video shows how to calculate the standard free energy change (delta G) at 298 Kelvin using the equilibrium partial pressure constant (Kp). It uses the formula delta G = -RT * ln K, where R is the gas constant and T is the temperature in Kelvin. The calculation results in a positive delta G, indicating a reactant-favored reaction, and the video concludes that delta G is positive when K is less than one.
📉 Determining the Equilibrium Constant from Delta G
The video concludes with a method to calculate the equilibrium constant (K) for a reaction given its standard free energy change at 298 Kelvin. It uses the relationship delta G = -RT * ln K and rearranges the formula to solve for K. The video emphasizes the importance of using the correct units for delta G and temperature and provides a step-by-step calculation that results in a large K value, indicating a product-favored reaction.
❄️ Analyzing Spontaneity in Physical Reactions
The video addresses the spontaneity of the physical reaction of liquid water turning into ice. It explains that the freezing of water is an exothermic process with a negative delta H and a negative delta S, as entropy decreases from liquid to solid. The video uses a decision table to show that the spontaneity of such a reaction depends on temperature: it is spontaneous at low temperatures, at equilibrium at the freezing point, and non-spontaneous at high temperatures.
Mindmap
Keywords
💡Gibbs Free Energy
💡Spontaneous Process
💡Equilibrium
💡Enthalpy Change (ΔH)
💡Entropy Change (ΔS)
💡Thermochemical Equations
💡Standard Free Energy Change
💡Non-Spontaneous Process
💡Reaction Quotient (Q)
💡Boiling Point
💡Equilibrium Constant (K)
Highlights
Gibbs free energy (ΔG) is used to determine if a process is spontaneous, at equilibrium, or non-spontaneous.
ΔG < 0 indicates a spontaneous process, ΔG = 0 indicates equilibrium, and ΔG > 0 indicates a non-spontaneous process.
The maximum work obtainable from a spontaneous process is equal to the change in free energy (ΔG).
To drive a non-spontaneous process, the minimum work required is equal to ΔG, accounting for energy losses due to friction.
The change in free energy (Gibbs free energy) is calculated as ΔH - TΔS, where ΔH is the enthalpy change, T is the temperature, and ΔS is the entropy change.
Units for ΔH and ΔG are kilojoules per mole, while ΔS is in joules per mole per kelvin, requiring conversion to kilojoules.
A negative ΔG value corresponds to a spontaneous process, as demonstrated through a calculation with ΔH = -46.5 kJ/mol and T = 298 K, resulting in ΔG = -109.7 kJ/mol.
For reactions, the enthalpy change (ΔH) and entropy change (ΔS) can be determined using the heat of formation values for reactants and products.
The calculation of ΔG for a reaction at 25°C involves using the formula ΔG = ΔH - TΔS with the given standard state values.
Thermochemical equations can be scaled by multiplying coefficients, which affects the energy change of the reaction but not the energy change per mole.
The non-standard ΔG value can be calculated using the reaction quotient (q) and the standard ΔG value in the equation ΔG = ΔG° + RT ln q.
When calculating boiling points, the system is at equilibrium, and ΔG = 0, which allows for the calculation of the boiling point temperature using ΔH and ΔS values.
The boiling point of bromine is estimated to be approximately 59°C using the given enthalpy of vaporization and standard entropy values.
The equilibrium constant (K) can be related to ΔG through the equation ΔG = -RT ln K, which is used to calculate K for given ΔG values.
The spontaneity of a reaction and its favorability towards products or reactants can be determined by the signs of ΔH and ΔS and the temperature.
The freezing of water into ice is a spontaneous process at low temperatures due to the exothermic nature and decrease in entropy.
Transcripts
Browse More Related Video

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry

Gibbs free energy and spontaneous reactions | Biology | Khan Academy

Gibbs free energy and spontaneity | Chemistry | Khan Academy
Chapter 6: K and Standard Gibbs Energy | CHM 214 | 051

Chapter 6: Gibbs Free Energy | CHM 214 | 050
[H2 Chemistry] 2021 Topic 5 Energetics 3
5.0 / 5 (0 votes)
Thanks for rating: