Entropy: Embrace the Chaos! Crash Course Chemistry #20
TLDRThe video explains that according to the second law of thermodynamics, the natural tendency of the universe is toward disorder, or entropy. It states that spontaneous chemical reactions increase randomness and require no outside energy input. The change in Gibbs free energy (G) determines if a reaction will occur spontaneously - a negative ΔG means the reaction is spontaneous. An endothermic reaction that absorbed heat yet froze surrounding water is analyzed; though counterintuitive, math shows the large increase in entropy drove the reaction, not the heat change. Josiah Willard Gibbs' equation relating enthalpy, entropy, temperature and free energy is introduced to calculate and confirm the reaction's spontaneity.
Takeaways
- 😟 Everything tends toward chaos and disorder due to probability.
- 😨 The second law of thermodynamics states that the disorder of the universe always increases.
- 😞 Increasing order requires energy and creates disorder elsewhere.
- 🤯 Entropy measures molecular randomness and disorder.
- 😎 Entropy helps explain how some reactions occur spontaneously without energy input.
- 😀 Gibbs free energy measures usable work available from a reaction.
- 🧠 Gibbs created an equation relating free energy to enthalpy and entropy changes.
- 📉 Negative Gibbs free energy change means a reaction is spontaneous.
- 🔍 Both enthalpy and entropy changes determine Gibbs free energy.
- 🎓 Understanding entropy and free energy explains counterintuitive spontaneous reactions.
Q & A
Why does the universe tend toward disorder?
-It's due to simple rules of probability - there are many more disorganized states than organized ones for things to be arranged in, so it's more likely for stuff to end up disorganized.
What does the Second Law of Thermodynamics state?
-It states that any spontaneous process increases the disorder or randomness of the universe.
What does 'spontaneous' mean in chemistry?
-In chemistry, 'spontaneous' doesn't refer to the speed of a reaction but rather means a reaction is thermodynamically capable of occurring without needing an input of external energy.
How is entropy calculated?
-Entropy is calculated by subtracting the sum of the reactant entropies from the sum of the product entropies, similar to how enthalpy change is calculated.
What does Gibbs free energy tell us?
-Gibbs free energy, or G, tells us how much energy in a system is free and available to do useful work. The change in Gibbs free energy (Delta G) can also tell us if a reaction is spontaneous.
When is a reaction considered enthalpy-driven?
-A reaction is enthalpy-driven when the heat transfer (enthalpy change) provides more free energy than the increase in disorder (entropy change).
When is a reaction considered entropy-driven?
-A reaction is entropy-driven when the increase in disorder (entropy change) provides more free energy than the heat transfer (enthalpy change).
Why was the example reaction with barium hydroxide spontaneous?
-The reaction had a large increase in entropy as it went from solid reactants to liquid and gaseous products, giving it enough free energy from increased disorder to be spontaneous.
How do you know if Delta G makes a reaction spontaneous?
-If Delta G is negative, the reaction releases free energy and is spontaneous. If Delta G is positive, the reaction requires free energy input to proceed.
What are the key things the video explains about thermodynamics?
-It explains entropy, spontaneity, Gibbs free energy, enthalpy vs. entropy driven reactions, and how to use Delta G to predict spontaneity.
Outlines
😟 The Second Law of Thermodynamics - Things Tend Toward Chaos and Disorder
Paragraph 1 discusses the inevitability of chaos and disorder in the universe. It introduces the Second Law of Thermodynamics which states that the entropy or disorder of the universe always increases for spontaneous processes. Examples are provided related to food molecules breaking down and house cleaning to illustrate how bringing order to one system results in greater disorder elsewhere.
🤔 An Entropy-Driven Chemical Reaction That Feels Cold
Paragraph 2 demonstrates an entropy-driven chemical reaction between barium hydroxide and ammonium chloride that absorbs heat from its surroundings, feels cold, and freezes wood. Calculations show the reaction increases entropy more than it changes enthalpy, confirming it is entropy-driven.
😎 Gibbs Free Energy Explains Spontaneity of Entropy-Driven Reactions
Paragraph 3 introduces Gibbs free energy, calculated from changes in enthalpy and entropy. The reaction from Paragraph 2 is shown to have negative Gibbs free energy, making it spontaneous even though it absorbed heat. This confirms reactions can happen spontaneously by increasing entropy rather than releasing heat.
Mindmap
Keywords
💡entropy
💡spontaneous process
💡Gibbs free energy
💡enthalpy
💡entropy-driven reaction
💡state function
💡second law of thermodynamics
💡disorder
💡standard entropy
💡standard free energy change
Highlights
The universe tends toward disorder due to simple rules of probability.
Entropy is a measure of molecular randomness or disorder.
The Second Law of Thermodynamics states that any spontaneous process increases the disorder of the universe.
In chemistry, "spontaneous" refers to reactions that can occur without added energy, not the speed of the reaction.
Entropy helps explain how some reactions can occur spontaneously even though they absorb energy.
Gibbs free energy measures the energy available to do useful work.
Gibbs created a formula to calculate free energy change from enthalpy and entropy changes.
Enthalpy change refers to heat transfer; entropy change refers to disorder created.
If enthalpy change outweighs TΔS, the reaction is enthalpy-driven. If TΔS outweighs enthalpy change, the reaction is entropy-driven.
Negative ΔG means the reaction releases free energy, indicating it is spontaneous.
The example reaction absorbed heat but was entropy-driven due to the large increase in disorder going from solids to liquids/gases.
The reaction was spontaneous because Gibbs free energy was negative, indicating free energy was released.
Even though the reaction absorbed heat, the large entropy change made it spontaneous without added energy.
Gibbs' formula shows whether a reaction is spontaneous based on the sign of ΔG.
The key things learned are entropy always increases, Gibbs' formula relates entropy and enthalpy to spontaneity, and the sign of ΔG indicates spontaneity.
Transcripts
Browse More Related Video

6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant | Organic Chemistry
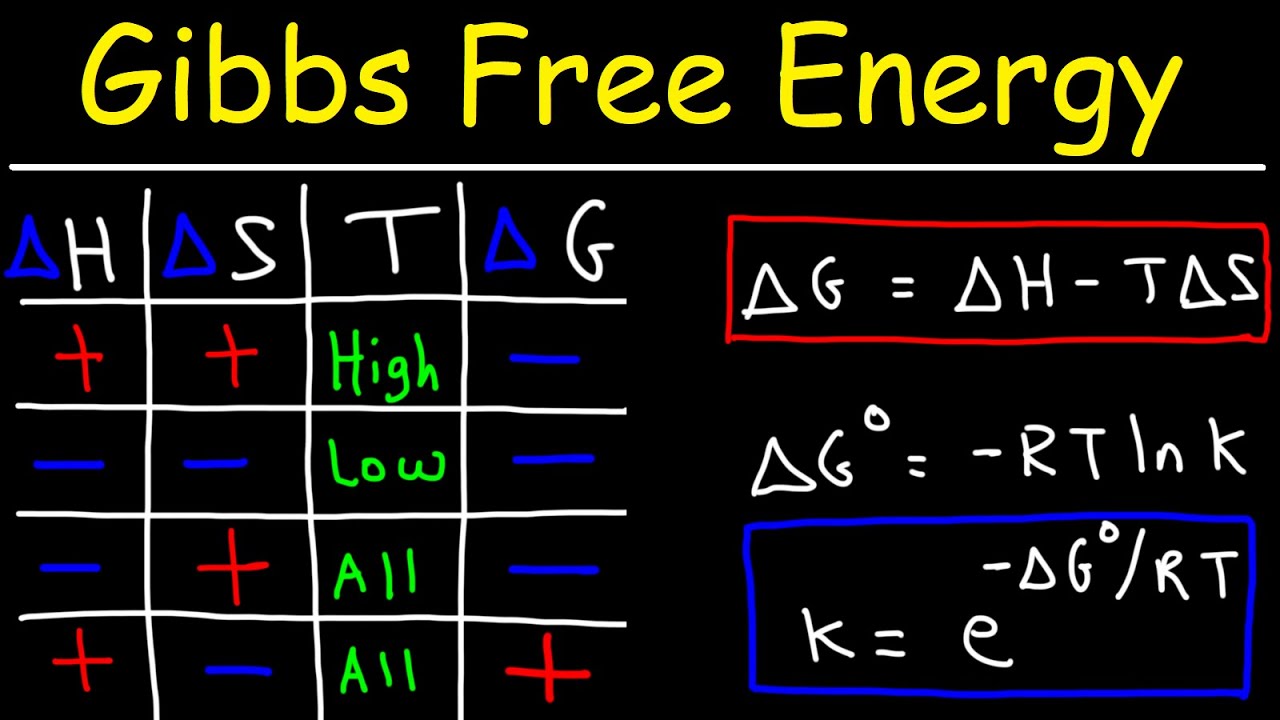
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K

Gibbs free energy example | Thermodynamics | Chemistry | Khan Academy

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry

16. Thermodynamics: Gibbs Free Energy and Entropy

Gibbs free energy and spontaneous reactions | Biology | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: