Second Law of Thermodynamics and entropy | Biology | Khan Academy
TLDRThis script explores the Second Law of Thermodynamics, emphasizing the principle that the universe's entropy always increases, reflecting a system's disorder. It uses the Hubble telescope's galaxy images to set a cosmic context, then delves into open and closed systems, using a campfire and an ice cooler as examples. The script illustrates how diffusion increases entropy, making processes irreversible, and explains that even seemingly reversible macroscopic events have microscopic irreversibilities due to entropy increase, all contributing to the universe's ever-growing disorder.
Takeaways
- 🌌 The Second Law of Thermodynamics states that the entropy of the universe only increases, which is a profound statement when considering the cosmos as depicted by the Hubble telescope image.
- 🔥 Entropy can be defined as the disorder of a system, often related to the number of possible states the system can take on.
- 🔄 The universe is considered the ultimate closed system with no external interactions, making it a key subject for the discussion of entropy increase.
- 🔥 An open system, like a campfire, interacts with its surroundings through heat and light, unlike a closed system which is isolated.
- 🧊 An ice cooler is an everyday example of an attempt to create a closed system, though not perfect as heat will eventually penetrate and melt the ice.
- 🔬 In research labs, there are better approximations of closed systems, but even these cannot be completely isolated from the universe.
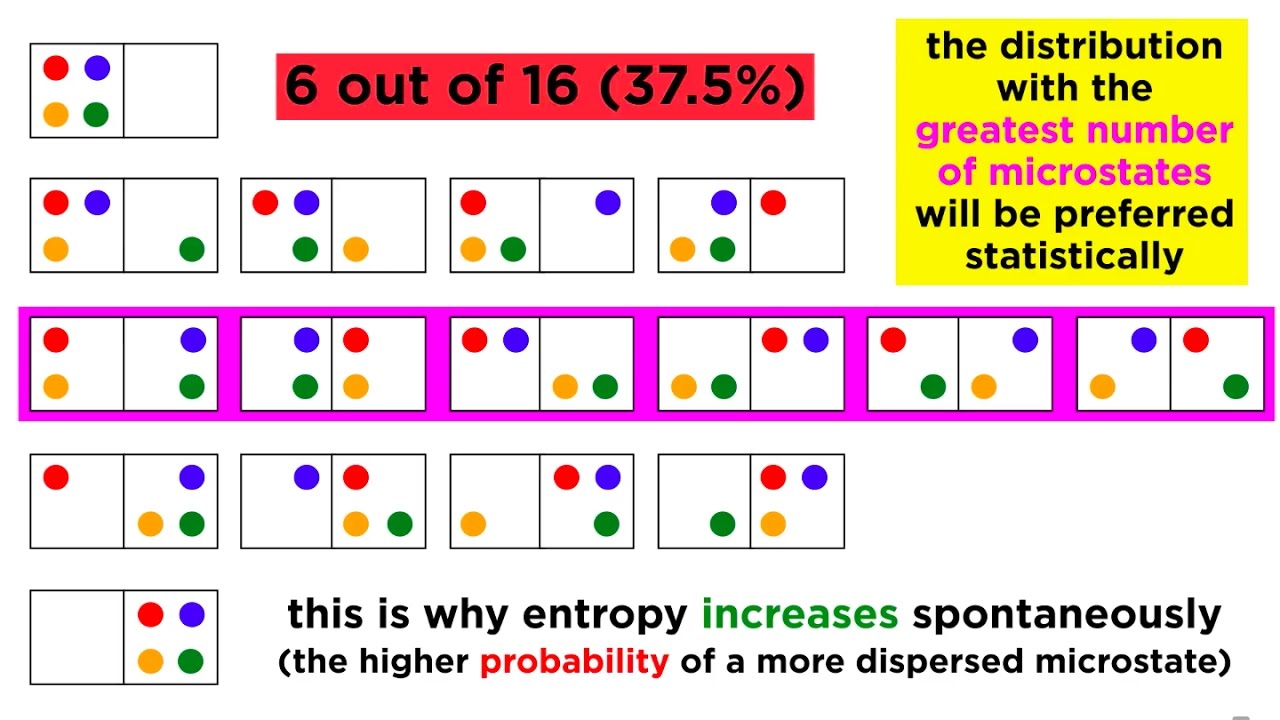
- 💡 The concept of diffusion is used to explain why entropy increases; particles spread out to occupy more space, leading to higher disorder and more possible states.
- ⏳ Irreversible processes, like diffusion, are characterized by an increase in entropy due to the extremely low probability of systems returning to a previous state with fewer states.
- 🎱 Macroscopic phenomena, such as billiard balls colliding, may seem reversible, but microscopic examination reveals entropy increase due to heat generation and molecular excitation.
- 🔁 Even processes considered approximately reversible in thermodynamics are not without an increase in entropy; there is no such thing as a process with zero entropy increase.
- 🌡️ Everyday activities and technology contribute to the universe's entropy by generating and releasing heat into the environment, thus increasing the number of possible states.
Q & A
What is the Second Law of Thermodynamics?
-The Second Law of Thermodynamics states that the entropy of the universe only increases, which means the disorder or the number of states a system can take on is always on the rise.
What is entropy and how is it related to the disorder of a system?
-Entropy is a measure of the disorder of a system, defined by the number of states that a system could potentially take on. Higher entropy indicates a higher level of disorder.
What is the difference between an open system and a closed system in thermodynamics?
-An open system is one that interacts thermodynamically with its surroundings, such as a campfire releasing heat and light. A closed system, on the other hand, is isolated from the rest of the universe and does not interact with its surroundings, although perfect isolation is hard to achieve.
Why is the universe considered the ultimate closed system?
-The universe is considered the ultimate closed system because there is nothing outside of it to interact with thermodynamically. It is fully contained within itself.
Can you provide an example of an everyday approximation of a closed system?
-An ice cooler is an everyday example of an approximation of a closed system. It attempts to isolate the inside from the outside environment through insulation, although it is not a perfect closed system as heat will eventually penetrate the insulation.
Why is the process of diffusion considered an irreversible process in terms of entropy?
-Diffusion is considered irreversible because it is highly unlikely that molecules will spontaneously gather back into a smaller volume after they have spread out. This process increases the number of possible states, thus increasing entropy.
What does it mean for a process to be irreversible?
-A process is considered irreversible when it is highly improbable that it could return to its initial state. This usually happens when a system transitions from a state of lower entropy to a state of higher entropy.
How does the concept of entropy relate to everyday life and the processes we observe?
-In everyday life, many processes contribute to the increase in entropy, such as heat generation from electronic devices, friction causing heat, and even the act of watching a video, all of which release heat into the universe, adding to the number of possible states.
Why do we perceive some macroscopic processes, like billiard balls colliding, as reversible?
-Macroscopic processes like billiard balls colliding may appear reversible because we can imagine the process happening in reverse. However, when examined at a microscopic level, these processes are irreversible due to the generation of heat and the increase in entropy.
What is the significance of the Second Law of Thermodynamics in understanding the universe's evolution?
-The Second Law of Thermodynamics is significant because it implies that the universe is moving towards a state of maximum disorder. This principle helps us understand the evolution of the universe and the direction of time.
How does the concept of entropy relate to the idea of energy dispersal?
-Entropy is directly related to energy dispersal. As energy spreads out in a system, the number of possible states increases, leading to an increase in entropy. This is evident in processes like diffusion and heat transfer.
Outlines
🌌 The Second Law of Thermodynamics and Cosmic Perspective
The paragraph introduces the Second Law of Thermodynamics, emphasizing its profound implications for the universe's entropy, which is always increasing. It uses the Hubble telescope's image of galaxies to set a cosmological context, explaining entropy as a measure of disorder or the number of possible states a system can take. The concept of closed and open systems is clarified through examples like a campfire and an ice cooler, illustrating the difficulty of creating a perfect closed system. The ultimate closed system is identified as the universe itself, highlighting the law's significance on a universal scale.
🔄 Irreversible Processes and the Inevitability of Entropy Increase
This paragraph delves into the concept of irreversible processes, using the example of gas diffusion in a container to illustrate how systems naturally progress from a state of lower to higher entropy. It explains that the transition from a confined state to one of greater disorder represents an increase in the system's possible states, thus increasing entropy. The paragraph further discusses the apparent reversibility of macroscopic processes, such as billiard balls colliding, versus the actual irreversible nature of these events when viewed on a microscopic level. It concludes by emphasizing that all interactions, from everyday activities to electronic devices, contribute to the universe's increasing entropy through the generation of heat.
Mindmap
Keywords
💡Second Law of Thermodynamics
💡Entropy
💡Universe
💡Open and Closed Systems
💡Irreversible Processes
💡Diffusion
💡Molecular Level
💡Heat
💡Friction
💡Reversible Processes
💡Macroscopic Level
Highlights
The Second Law of Thermodynamics states that the entropy of the universe only increases, signifying a profound principle.
Entropy is defined as the disorder of a system, related to the number of possible states it can take on.
The universe is considered the ultimate closed system with no external thermodynamic interactions.
Review of open and closed systems using the example of a campfire and an ice cooler.
Difficulties in creating a true closed system in everyday life, with the universe as the only perfect example.
Diffusion is used as an example to explain the increase in entropy over time.
The process of diffusion leads to higher entropy as particles spread out and occupy more space.
Irreversible processes are characterized by an increase in entropy, with low probability of returning to the initial state.
Macroscopic reversible processes are shown to be approximately reversible, but entropy still increases at a microscopic level.
Microscopic examination reveals that even in seemingly reversible processes, entropy increases due to heat generation and molecular excitation.
The concept that the universe is constantly undergoing irreversible processes, contributing to its increasing entropy.
Examples of everyday activities and technology that contribute to the universe's entropy by releasing heat.
The heat generated from friction, motion, and electronic activity adds to the universe's possible states.
The Second Law of Thermodynamics implies that the disorder or number of states in the universe only increases over time.
The explanation of why smoke does not naturally condense back into a shaped particle, illustrating the irreversibility of entropy increase.
The odds of observing a reversal of entropy in systems with a vast number of molecules are practically zero.
The significance of the Second Law of Thermodynamics in understanding the fundamental direction of natural processes and the flow of energy.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: