Second Law of Thermodynamics
TLDRIn this AP Physics essentials video, Mr. Andersen explains the second law of thermodynamics, focusing on the concept of entropy. Entropy is defined as a measure of disorder in a system, and the law states that in a closed system, entropy never decreases but always increases over time. This principle applies to both reversible and irreversible processes, with the latter being characterized by a rise in entropy. Andersen uses the analogy of videos played in forward and reverse directions to illustrate the difference between the two types of processes. He also clarifies that while it may seem counterintuitive, the creation of ordered systems like computers involves increasing the disorder of the surroundings, thus adhering to the second law. The video concludes by emphasizing the importance of understanding the qualitative nature of entropy and its role as a state function in the context of the second law of thermodynamics.
Takeaways
- 🔄 The first law of thermodynamics states that energy cannot be created or destroyed, while the second law relates to entropy, which is a measure of disorder in a process.
- 🔍 Entropy is the amount of disorder, and it is more likely for a system to become more disordered than for it to spontaneously organize itself.
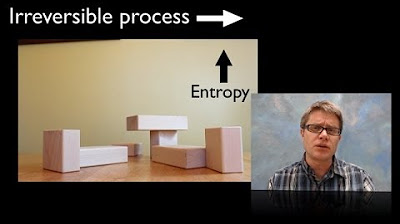
- ↔️ Processes can be reversible, meaning they can occur in either direction without a change in entropy, or irreversible, where entropy increases over time.
- 📏 Entropy is a state function, meaning it is measured at a specific point or state and indicates the level of disorder or chaos in a system.
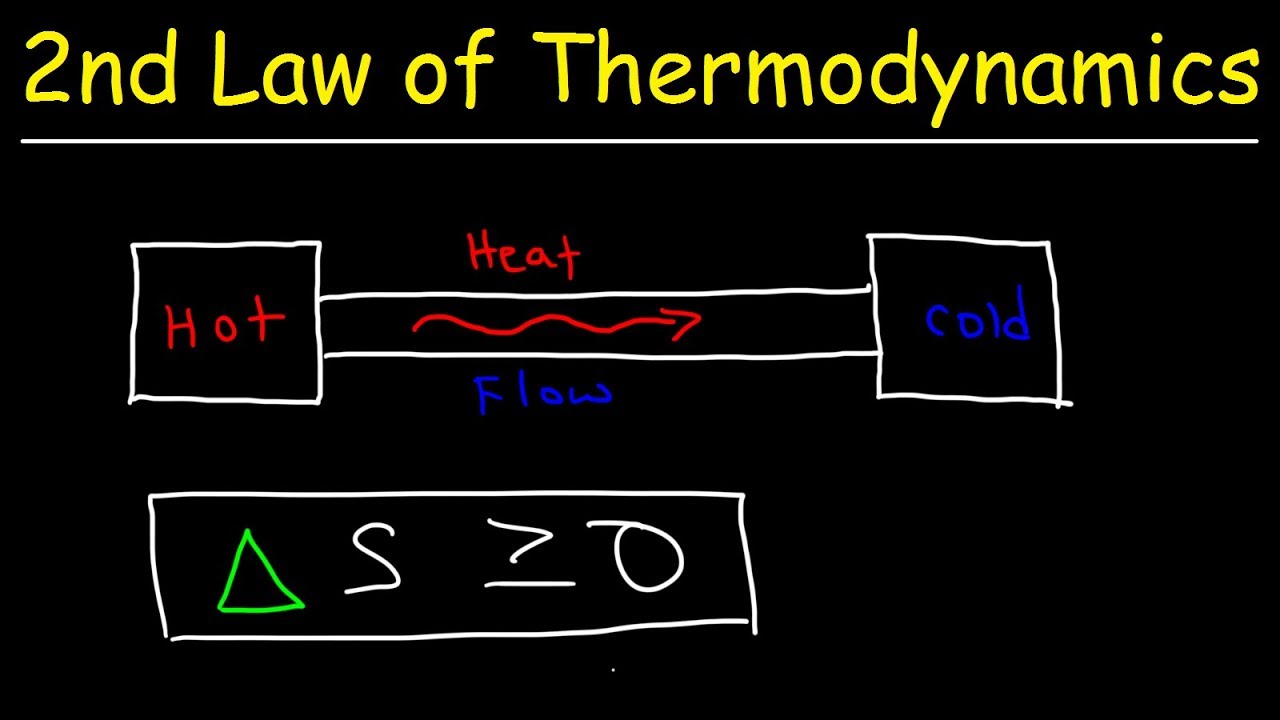
- 🚫 In a closed system, entropy never decreases and always increases over time, which is a fundamental principle of the second law of thermodynamics.
- 🌌 The concept of entropy increase over time applies to the universe as a whole, suggesting a universal progression towards greater disorder.
- 🎥 The provided videos illustrate the difference between reversible and irreversible processes, with the latter being statistically improbable to occur in reverse.
- ⬆️ In an irreversible process, entropy increases, which can also be thought of as the direction of time's arrow, moving towards greater disorder.
- 🤔 Despite the second law, complex and ordered structures like computers and videos can exist, but their creation results in a more significant increase in entropy elsewhere in the system.
- 🔩 The creation of order in a system (non-closed) is possible by increasing the disorder of the surroundings, which aligns with the overall increase in entropy over time.
- ⏳ Entropy and the second law of thermodynamics are essential for understanding the natural progression of systems towards a state of greater disorder.
- 📚 AP Physics students are not required to quantify entropy but should understand its qualitative nature and its implications for the direction of natural processes.
Q & A
What is the second law of thermodynamics?
-The second law of thermodynamics relates to entropy, which is a measure of the amount of disorder in a process. It states that in a closed system, entropy will never decrease and will always increase over time.
What is entropy?
-Entropy is a measure of disorder in a system. It can also be thought of as the lack of energy available to do work or as a representation of the system's organization or chaos.
How does the second law of thermodynamics differentiate between reversible and irreversible processes?
-In a reversible process, the amount of entropy does not change and the process can go either way. In an irreversible process, the amount of entropy increases over time, indicating a unidirectional flow.
Why is entropy considered a state function?
-Entropy is a state function because it is measured at one point or state in time, reflecting the disorder at that specific moment without regard to how the system reached that state.
What does it mean for a process to be irreversible?
-An irreversible process is one in which the entropy increases over time. It is statistically improbable or impossible for such a process to occur in the reverse direction.
How can we visualize the concept of entropy in terms of time?
-Entropy can be visualized as 'time's arrow,' moving in the direction of time, especially in irreversible processes where disorder increases.
What is the implication of the second law of thermodynamics for the universe?
-According to the second law of thermodynamics, the entropy of the universe will continue to increase over time, implying a continuous rise in disorder at a universal level.
Can you give an example of an irreversible process from the script?
-An example of an irreversible process from the script is the video where milk is seen moving outside of a cup. It is highly improbable for milk to spontaneously move back into the cup.
How does the script explain the creation of order in systems like a computer or a video?
-The script explains that while a computer or a video may appear to have order, they are not closed systems. Their order is achieved by increasing the disorder in the surrounding environment, which is consistent with the second law of thermodynamics.
What is the significance of the second law of thermodynamics in understanding the direction of time?
-The second law of thermodynamics is significant in understanding the direction of time because it dictates that entropy in a closed system will always increase, thus providing a directionality to time's progression.
How does the script illustrate the concept of entropy increasing over time?
-The script illustrates this concept by showing videos played in forward and reverse directions, and by discussing the natural progression from a state of lower disorder to higher disorder over time.
Outlines
🔄 Introduction to the Second Law of Thermodynamics
Mr. Andersen introduces the second law of thermodynamics, contrasting it with the first law which deals with energy conservation. He explains entropy as a measure of disorder in a system, using the example of increasing disorder by writing the word 'entropy'. He distinguishes between reversible and irreversible processes in terms of entropy change, with the latter leading to an increase in entropy over time. Entropy is described as a state function that measures disorder at a point in time and can also be thought of as the lack of energy available to do work. The video emphasizes that in a closed system, entropy never decreases and always increases over time, which is a fundamental concept in understanding the second law of thermodynamics.
Mindmap
Keywords
💡Second Law of Thermodynamics
💡Entropy
💡Reversible Process
💡Irreversible Process
💡State Function
💡Disorder
💡Energy
💡Closed System
💡Time's Arrow
💡Chaos
💡Organization
Highlights
The second law of thermodynamics relates to entropy, which is a measure of disorder in a process.
Entropy is the likelihood of a system falling apart rather than spontaneously reassembling.
Processes can be reversible, with no change in entropy, or irreversible, with entropy increasing over time.
Entropy is a state function, measured at one point in time, indicating the system's disorder or chaos.
In a closed system, entropy never decreases and always increases over time.
The universe's entropy is expected to increase over time, indicating a unidirectional flow of time.
Video examples demonstrate the difference between reversible and irreversible processes.
Reversible processes are equally likely to occur in either direction, unlike irreversible processes.
Irreversible processes are characterized by an increase in entropy and a clear direction of time's arrow.
Entropy can be thought of as the lack of energy available to do work, indicating system organization.
In the context of a closed system, entropy is a unidirectional measure, always increasing with time.
The creation of ordered systems like computers and videos requires increasing disorder in the surroundings.
Over time, the entropy of the environment increases as local order is created at the expense of the surroundings.
The second law of thermodynamics and the concept of entropy connect qualitatively, explaining the direction of time and the increase of disorder.
Understanding entropy helps to explain the natural progression from order to disorder over time in closed systems.
The video concludes by emphasizing the importance of recognizing entropy as a key concept in thermodynamics.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: