Entropy - 2nd Law of Thermodynamics - Enthalpy & Microstates
TLDRThis educational video script delves into the concept of entropy, explaining spontaneous and non-spontaneous processes. It illustrates how processes like a ball rolling downhill are spontaneous, contrasting with cleaning a room, which is not. The script explores the relationship between entropy, probability, and the second law of thermodynamics, emphasizing that spontaneous processes tend to increase the universe's entropy. It uses examples like gas distribution and coin tosses to clarify how disordered states are more probable, leading to higher entropy. The script also discusses how to determine if a process is spontaneous based on heat flow and its impact on system and surroundings' entropy.
Takeaways
- 🔄 A spontaneous process is one that occurs naturally without external intervention, while a non-spontaneous process requires energy input to occur.
- ⛰️ The rolling of a ball down a hill is a spontaneous process, whereas rolling it up would be non-spontaneous.
- 🌿 Rusting of iron is a spontaneous process when exposed to air and water, happening naturally over time.
- 🧹 Cleaning a room is non-spontaneous as it requires work and does not happen by itself; rooms tend to become disorganized without intervention.
- 🔥 Heat naturally flows from hot to cold, which is a spontaneous process, but requires energy to reverse this flow, as seen in refrigerators.
- 💧 Gasoline reacts with air to produce carbon dioxide and water, a spontaneous process that can be accelerated with a spark.
- 📈 The concept of positional probability relates to the number of microstates a system can occupy, with higher numbers indicating a more probable state.
- 🔄 Entropy is associated with the number of microstates; a system tends to move towards a state of higher entropy or greater disorder.
- 🎲 Flipping coins illustrates the principle of entropy, where a disorganized mix of heads and tails is more probable than all heads or all tails.
- ⚖️ The second law of thermodynamics states that the entropy of the universe increases for spontaneous processes and decreases for non-spontaneous ones.
- 🌡️ In an exothermic process, heat flows from the system to the surroundings, increasing the entropy of the surroundings and decreasing the system's entropy.
Q & A
What is a spontaneous process?
-A spontaneous process is one that can occur without any outside intervention. It happens naturally on its own.
Why is rolling a ball down a hill considered a spontaneous process?
-Rolling a ball down a hill is a spontaneous process because it will naturally occur on its own without any external force or energy input.
What does it mean for a process to be non-spontaneous?
-A non-spontaneous process is one that does not happen on its own and requires energy or work to be initiated or to continue.
How does the rusting of iron in the presence of air and water illustrate a spontaneous process?
-The rusting of iron is a spontaneous process because it will naturally occur over time when iron is exposed to air and water, without any additional intervention.
Why is cleaning a bedroom considered a non-spontaneous process?
-Cleaning a bedroom is non-spontaneous because it requires work and energy to organize and tidy up the space, which does not happen naturally on its own.
What is the relationship between entropy and the probability of a state?
-Entropy is associated with the probability of a state. Higher entropy indicates a higher number of microstates, making a state more probable or likely to occur.
Why do spontaneous processes tend to increase the disorder of a system?
-Spontaneous processes tend to increase disorder because disordered states generally have a higher number of microstates, making them more probable than ordered states.
How does the tossing of four coins illustrate the concept of entropy and probability?
-When tossing four coins, the most probable outcome is a mix of heads and tails, which represents a higher entropy state compared to the less probable outcomes of all heads or all tails.
What does the second law of thermodynamics state about the entropy of the universe in relation to spontaneous processes?
-The second law of thermodynamics states that for a spontaneous process, the change in the entropy of the universe is positive, indicating an increase in overall disorder.
How does the concept of microstates relate to the entropy of a system?
-The number of microstates in a system is directly related to its entropy. A system with more microstates has higher entropy, reflecting a greater degree of disorder or randomness.
Why is it improbable for all molecules in a container to be on one side, according to the script?
-It is improbable for all molecules to be on one side of a container because this state has fewer microstates, indicating a lower probability compared to a distribution that maximizes microstates, such as an even spread of molecules.
Outlines
🔄 Understanding Spontaneity and Entropy
This paragraph introduces the concept of spontaneous and non-spontaneous processes in relation to entropy. It explains that a spontaneous process occurs naturally without external influence, while a non-spontaneous process requires energy input. The paragraph uses the analogy of a ball rolling down a hill to illustrate a spontaneous process and the difficulty of rolling it back up as non-spontaneous. It also discusses the spontaneous rusting of iron and the non-spontaneous act of cleaning a room, emphasizing that natural processes tend toward increased disorder. The paragraph concludes by identifying the cleaning of a room as a non-spontaneous process due to the work required to achieve order.
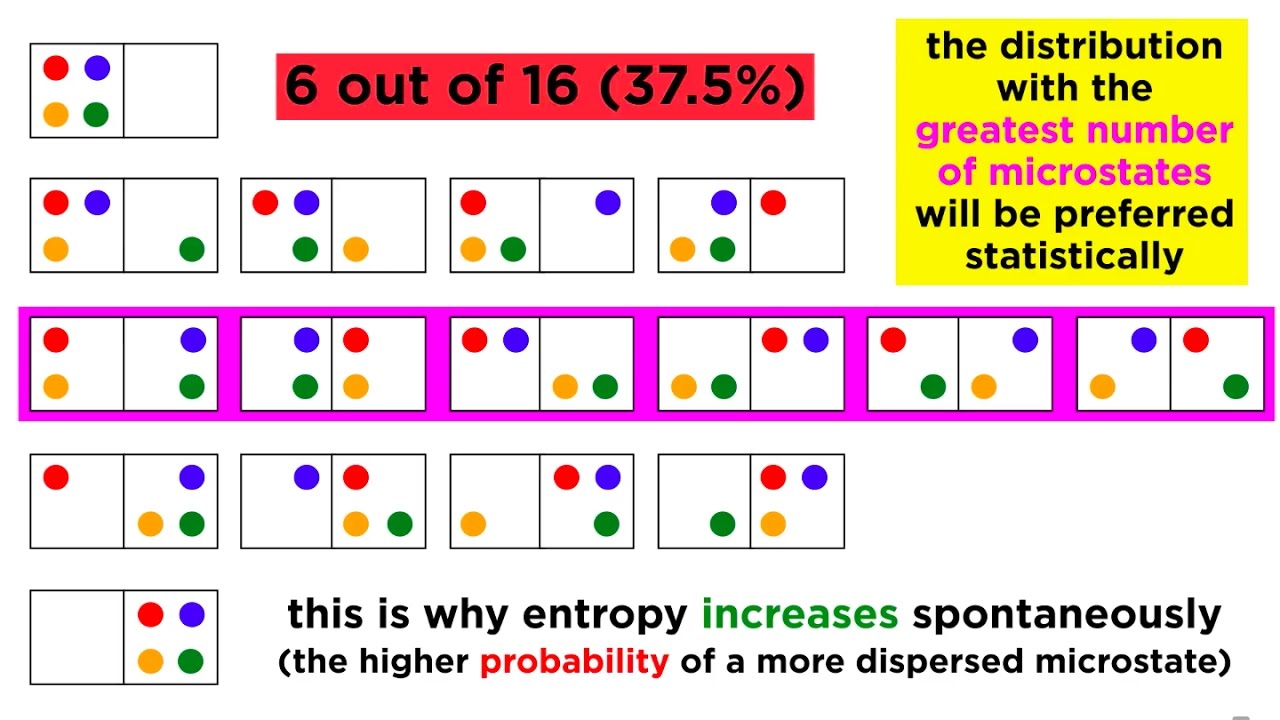
📈 Calculating Microstates and Positional Entropy
The second paragraph delves into calculating the number of microstates to determine the positional probability and entropy of different systems. It explains that gases naturally expand to fill containers, representing a state of high positional probability. The paragraph provides examples of calculating microstates for different systems, such as one with all molecules on one side of a container, another with a single molecule on one side, and a system with an equal distribution of molecules. It concludes that system B, with an equal distribution of molecules, has the highest number of microstates and thus the highest positional entropy, making it the most probable state.
🎲 The Probability Driving Spontaneous Processes
This paragraph explores the idea that the driving force behind spontaneous processes is an increase in probability, with disordered states being more probable than ordered ones. It uses the example of organizing and disorganizing a room to illustrate this point and explains that natural processes tend toward higher entropy or more disorder. The paragraph also discusses the tossing of coins to demonstrate how the probability of a disorganized outcome (a mix of heads and tails) is higher than that of an organized outcome (all heads or all tails). It concludes by emphasizing that the entropy of a system increases as the number of microstates increases, aligning with the tendency of natural processes to move toward higher probability and disorder.
🔢 Entropy and Coin Tossing Probabilities
The fourth paragraph examines the relationship between entropy and the probability of disorganized states using the example of coin tossing. It explains how the probability of achieving an organized state (all heads or all tails) decreases as the number of coins increases, while the probability of a disorganized state (a mix of heads and tails) increases. The paragraph calculates the probabilities for different numbers of coins and illustrates that with a large number of coins, such as Avogadro's number, the chance of all coins landing on one side becomes negligible. It concludes by reinforcing the idea that the probability of a disorganized state increases with the number of particles involved, aligning with the second law of thermodynamics.
⚙️ Entropy and the Second Law of Thermodynamics
This paragraph discusses the second law of thermodynamics in the context of entropy changes in spontaneous, reversible, and non-spontaneous processes. It explains that for a spontaneous process, the entropy change of the universe is positive, indicating an increase in disorder. For a process at equilibrium, the entropy change is zero, and for a non-spontaneous process, the entropy change is negative, indicating a decrease in disorder. The paragraph also addresses common misconceptions about the entropy changes of the system and surroundings, emphasizing that these changes can be positive or negative and are not always in the same direction. It concludes by identifying statement D as the true statement that the entropy change of the universe is negative for a non-spontaneous process.
🌡️ Entropy Changes in Exothermic Processes
The final paragraph examines the entropy changes in an exothermic spontaneous process, focusing on the direction of heat flow and its impact on the entropy of the system and surroundings. It explains that during an exothermic process, heat flows from the hot system to the colder surroundings, leading to an increase in entropy for the surroundings and a decrease in entropy for the system. The paragraph also discusses the relationship between temperature and entropy, noting that higher temperatures correspond to higher entropy values. It concludes by evaluating statements about the entropy changes in the system, surroundings, and universe, identifying statements B, C, and D as true, indicating that the entropy of the surroundings increases, the entropy of the universe increases for a spontaneous process, and the entropy of the system decreases.
Mindmap
Keywords
💡Spontaneous process
💡Non-spontaneous process
💡Entropy
💡Microstates
💡Probability
💡Second law of thermodynamics
💡Exothermic process
💡Endothermic process
💡Equilibrium
💡Disorder
💡Organization
Highlights
A spontaneous process is one that occurs naturally without outside intervention.
Non-spontaneous processes require energy input to occur.
A ball rolling down a hill exemplifies a spontaneous process.
Cleaning a room is a non-spontaneous process as it requires work.
Heat naturally flows from hot to cold, illustrating a spontaneous process.
Gasoline's reaction with air to produce carbon dioxide is spontaneous but slow.
The driving force for a spontaneous process is an increase in probability.
Disordered states are more probable than ordered states, leading to higher entropy.
The concept of microstates is introduced to explain the most probable state of a system.
Calculating the number of microstates can determine the system's positional entropy.
The most probable state of a system has the highest number of microstates.
Flipping coins illustrates the concept of increasing disorder and decreasing probability of an organized outcome.
The second law of thermodynamics relates to the entropy of the universe in spontaneous and non-spontaneous processes.
The entropy of the universe is the sum of the system's and surroundings' entropy changes.
Spontaneity of a process is determined by comparing the magnitudes of entropy changes in the system and surroundings.
An exothermic process results in a decrease in the system's entropy and an increase in the surroundings' entropy.
The entropy of the universe always increases for a spontaneous process according to the second law of thermodynamics.
Transcripts
Browse More Related Video
Second Law of Thermodynamics - Heat Energy, Entropy & Spontaneous Processes
What is entropy? - Jeff Phillips
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates

6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant | Organic Chemistry

Understanding Second Law of Thermodynamics !

Entropy: Embrace the Chaos! Crash Course Chemistry #20
5.0 / 5 (0 votes)
Thanks for rating: