GCSE Chemistry - Alkanes: properties & combustion #52
TLDRThis video explores the properties of alkanes, a homologous series of hydrocarbons with carbon and hydrogen atoms, and no double bonds. It explains how boiling points, volatility, and flammability vary with carbon chain length. Alkanes with shorter chains are more volatile and flammable, making them excellent fuels. The video also covers the concept of complete combustion, where hydrocarbons react with oxygen to produce carbon dioxide and water, releasing energy. It demonstrates how to write and balance chemical equations for the combustion of alkanes, such as propane and nonane, highlighting the importance of oxygen for complete combustion.
Takeaways
- 🔬 Alkanes are a homologous series of hydrocarbons with only single bonds between carbon and hydrogen atoms.
- 📝 The first four alkanes in the series were named in the previous video, but this video focuses on alkane properties and combustion equations.
- 🌡 Boiling points of alkanes increase with the length of the carbon chain; shorter alkanes are gases at room temperature, while longer ones are liquids or solids.
- 💨 Shorter alkanes are more volatile and evaporate more easily due to their lower boiling points.
- 🍯 Longer alkanes are more viscous, exhibiting a thicker consistency similar to honey.
- 🔥 Shorter alkanes are more flammable and easier to ignite or burn, which is a desirable trait for fuels.
- 🔥 Combustion of hydrocarbons like alkanes is a primary use due to the energy released when they react with oxygen.
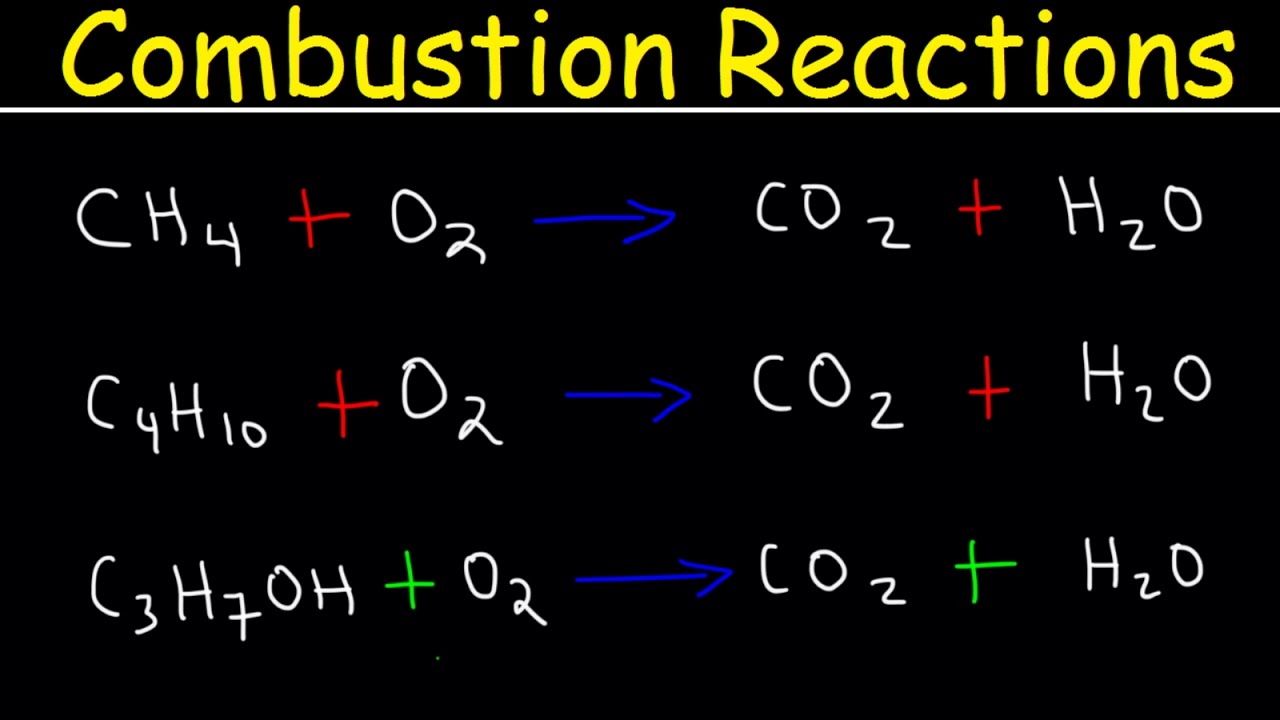
- 🔄 Complete combustion occurs when a hydrocarbon reacts with oxygen to form carbon dioxide and water, an exothermic reaction.
- ⚖️ Balancing chemical equations for the combustion of alkanes, such as propane (C3H8), is essential for understanding the stoichiometry of the reaction.
- 📚 For example, the balanced equation for the complete combustion of propane involves five O2 molecules to react with three carbon atoms and eight hydrogen atoms to produce three CO2 and four H2O molecules.
- 📝 The video also demonstrates how to write and balance the combustion equation for nonane (C9H20), highlighting the importance of balancing oxygen atoms in the reaction.
Q & A
What is a homologous series of hydrocarbons?
-A homologous series of hydrocarbons is a group of compounds that have similar chemical properties and structures, differing from each other by a constant unit, typically a CH2 group.
What are the characteristics of alkanes in terms of their chemical bonds?
-Alkanes are characterized by having only single bonds between carbon and hydrogen atoms, with no double bonds present in their molecular structure.
How does the boiling point of alkanes change with the length of their carbon chain?
-The boiling point of alkanes increases with the length of their carbon chain. Shorter alkanes have lower boiling points and are gases at room temperature, while longer alkanes can be liquids or even solids.
Why are shorter alkanes more volatile than longer ones?
-Shorter alkanes are more volatile because they have lower boiling points, which means they evaporate more easily compared to longer alkanes with higher boiling points.
What does it mean for an alkane to be more viscous, and how does this property relate to the length of the carbon chain?
-A more viscous alkane is thicker and stickier, similar to honey. This property increases with the length of the carbon chain, as longer chains tend to have higher molecular weights and stronger intermolecular forces.
How does the flammability of alkanes relate to the length of their carbon chains?
-Shorter alkanes are more flammable, meaning they are easier to ignite or burn. This is due to their lower boiling points and higher volatility.
What is the main use of hydrocarbons like alkanes?
-The main use of hydrocarbons like alkanes is as fuel, as they release a significant amount of energy when burned with oxygen.
What is complete combustion, and what are its products?
-Complete combustion is a reaction where a hydrocarbon reacts with oxygen to form carbon dioxide (CO2) and water (H2O), releasing a large amount of energy in the process.
How can you write a balanced equation for the complete combustion of propane (C3H8)?
-To write a balanced equation for the complete combustion of propane, you would react C3H8 with O2 to form CO2 and H2O, ensuring that the number of atoms for each element is the same on both sides of the equation. The balanced equation is C3H8 + 5O2 → 3CO2 + 4H2O.
What is the molecular formula for nonane, and how can you write its balanced combustion equation?
-The molecular formula for nonane is C9H20. To write its balanced combustion equation, you would react C9H20 with O2 to form 9CO2 and 10H2O, and then balance the oxygen atoms, resulting in C9H20 + 14O2 → 9CO2 + 10H2O.
What is the significance of the length of a hydrocarbon's carbon chain in terms of its properties and uses?
-The length of a hydrocarbon's carbon chain affects its boiling point, volatility, viscosity, and flammability. Shorter chains have lower boiling points, making them more volatile and flammable, which makes them suitable as fuels for combustion.
Outlines
🔥 Properties and Combustion of Alkanes
This paragraph introduces alkanes as a homologous series of hydrocarbons with carbon and hydrogen atoms and no double bonds. It discusses the properties of alkanes, emphasizing how these properties change with the length of the carbon chain. Shorter alkanes have lower boiling points, making them more volatile and flammable, and are typically gases at room temperature. Longer alkanes are more viscous and can be liquids or solids. The paragraph also explains combustion reactions, highlighting complete combustion as an exothermic reaction that produces carbon dioxide and water, releasing energy. The importance of balancing chemical equations for alkanes like propane and nonane during combustion is also covered.
🌟 Conclusion on Alkanes and Combustion
The concluding paragraph summarizes the key points of the video, emphasizing the dependency of alkanes' properties on the length of their carbon chains. It reiterates that the shortest hydrocarbons have the lowest boiling points, making them volatile and flammable, which is ideal for use as fuels. The paragraph also reinforces the concept of combustion as the process of using hydrocarbons as fuel, with complete combustion occurring only when there is sufficient oxygen available, resulting in the production of carbon dioxide and water and the release of significant energy.
Mindmap
Keywords
💡Alkanes
💡Homologous Series
💡Boiling Point
💡Volatile
💡Viscosity
💡Flammability
💡Combustion Reactions
💡Complete Combustion
💡Exothermic Reaction
💡Oxidation
💡Balancing Equations
Highlights
Alkanes are a homologous series of hydrocarbons with only carbon and hydrogen atoms and no double bonds.
The first four alkanes in the series have been named in the previous video.
Properties of alkanes vary with the length of the carbon chain.
Boiling points increase with the length of the carbon chain in alkanes.
Shorter alkanes with fewer than four carbons are gases at room temperature.
Longer alkanes with more than four carbons are liquids, and very long chains can be solids.
Shorter alkanes are more volatile due to their lower boiling points.
Longer alkanes are more viscous, similar to honey.
Shorter alkanes are more flammable and easier to ignite.
Combustion reactions of hydrocarbons release energy when burned with oxygen.
Complete combustion forms carbon dioxide and water, an exothermic reaction.
Hydrogen and carbon in hydrocarbons are oxidized during combustion.
Balancing chemical equations is necessary for understanding complete combustion.
Propane (C3H8) combustion equation is an example of balancing in chemistry.
Nonane (C9H20) combustion equation demonstrates balancing with a longer hydrocarbon.
The properties of hydrocarbons are dependent on the carbon chain length, affecting their use as fuels.
Short hydrocarbons are ideal fuels due to their volatility and flammability.
Combustion as a process for hydrocarbons requires sufficient oxygen for complete combustion.
The video concludes with an overview of alkane properties and combustion.
Transcripts
Browse More Related Video
Balancing Combustion Reactions

Quick Revision - Alkanes

Chemical Reactions (3 of 11) Combustion Reactions, An Explanation

Predicting Products | Combustion Reactions

Hydrocarbons - Aliphatic vs Aromatic Molecules - Saturated & Unsaturated Compounds

Visualize & Name Organic Compounds in Organic Chemistry - [1-2-32]
5.0 / 5 (0 votes)
Thanks for rating: