Predicting Products | Combustion Reactions
TLDRThis video script offers an educational insight into predicting the products of combustion reactions. It begins by defining combustion as the process of starting a fire by burning a substance with oxygen, typically initiated by a spark. The focus is on hydrocarbons, which are molecules composed solely of hydrogen and carbon, such as methane (CH4) or propane (C3H8). The script emphasizes that the reaction of any hydrocarbon with oxygen gas (O2), often symbolized by a fire, will always result in the formation of carbon dioxide and water. The video aims to teach viewers how to identify combustion reactions and distinguish them from other types such as single replacement reactions. It guides through practice problems, illustrating how to recognize hydrocarbons and predict the standard products of combustion, regardless of the specific hydrocarbon involved. The summary encourages viewers to pause and attempt the problems independently before revealing the answers, fostering active learning and engagement.
Takeaways
- 🔥 **Combustion Definition**: Combustion involves starting a fire by burning a substance with oxygen and a spark.
- ⛓ **Hydrocarbons**: Hydrocarbons are molecules composed solely of hydrogen and carbon atoms, which can be a chain of any length.
- 🔍 **Recognizing Hydrocarbons**: To identify a hydrocarbon, look for a compound containing only carbon and hydrogen.
- 🔬 **Combustion Reaction**: A combustion reaction specifically involves a hydrocarbon reacting with oxygen gas (O2) to produce fire.
- 🌐 **Combustion Products**: The reaction between any hydrocarbon and oxygen always results in the formation of carbon dioxide (CO2) and water (H2O).
- 🚫 **Not Single Replacement**: Despite similarities, combustion should not be confused with single replacement reactions, as they yield different products.
- ✅ **Identifying Combustion**: To determine if a reaction is combustion, check for the presence of a hydrocarbon and its interaction with oxygen.
- ❌ **Non-Combustion Reactions**: Reactions that do not involve a hydrocarbon and oxygen, such as with water or carbon dioxide, are not combustion reactions.
- 🧐 **Double Replacement Clarification**: Some reactions may resemble combustion but are actually double replacement reactions if water is involved instead of oxygen.
- 📝 **Balancing Equations**: Although the focus is on predicting products, it's important to note that combustion reaction equations need to be balanced.
- 📚 **Practice for Recognition**: The script provides practice problems to help viewers distinguish between combustion and non-combustion reactions.
Q & A
What does the term 'combustion' mean in the context of chemical reactions?
-Combustion refers to the process of starting a substance on fire by burning it with oxygen, typically initiated by a spark.
What is a hydrocarbon?
-A hydrocarbon is a compound consisting of hydrogen and carbon atoms bonded together, forming a chain of carbon atoms with hydrogen atoms attached.
What are the typical products formed when a hydrocarbon undergoes combustion?
-The typical products of a hydrocarbon combustion reaction are carbon dioxide (CO2) and water (H2O).
How is combustion different from a single replacement reaction?
-Although combustion can technically be considered a single replacement reaction, in the context of predicting products, it is treated separately because it always results in carbon dioxide and water, regardless of the specific hydrocarbon involved.
What is the role of oxygen in a combustion reaction?
-Oxygen is a reactant in a combustion reaction, combining with a hydrocarbon to produce carbon dioxide and water upon ignition.
Why is it important to recognize hydrocarbons when predicting combustion products?
-Recognizing hydrocarbons is crucial because the presence of a hydrocarbon and oxygen in a reaction indicates combustion, which consistently yields carbon dioxide and water as products.
How can you identify if a given reaction is a combustion reaction just by looking at the reactants?
-A reaction is a combustion reaction if it involves a hydrocarbon (a molecule containing only carbon and hydrogen) and oxygen gas (O2) as reactants.
What is the significance of balancing chemical equations in combustion reactions?
-Balancing chemical equations ensures that the number of atoms for each element on the reactant side is equal to the number on the product side, adhering to the law of conservation of mass.
Why does the presence of a spark facilitate the start of a combustion reaction?
-A spark provides the necessary energy to initiate the reaction between the hydrocarbon and oxygen, allowing the combustion process to begin.
What happens to a fire when the supply of oxygen is cut off?
-When the supply of oxygen is cut off, the fire will cease to burn because oxygen is required for combustion to continue.
Can you predict the products of a combustion reaction without knowing the specific hydrocarbon involved?
-Yes, you can predict the products as carbon dioxide and water for any hydrocarbon undergoing combustion, regardless of the specific hydrocarbon.
What is the difference between a combustion reaction and a double replacement reaction?
-A combustion reaction involves a hydrocarbon and oxygen to form carbon dioxide and water, while a double replacement reaction involves the exchange of ions between two compounds without necessarily involving oxygen or producing carbon dioxide and water.
Outlines
🔥 Understanding Combustion and Hydrocarbons
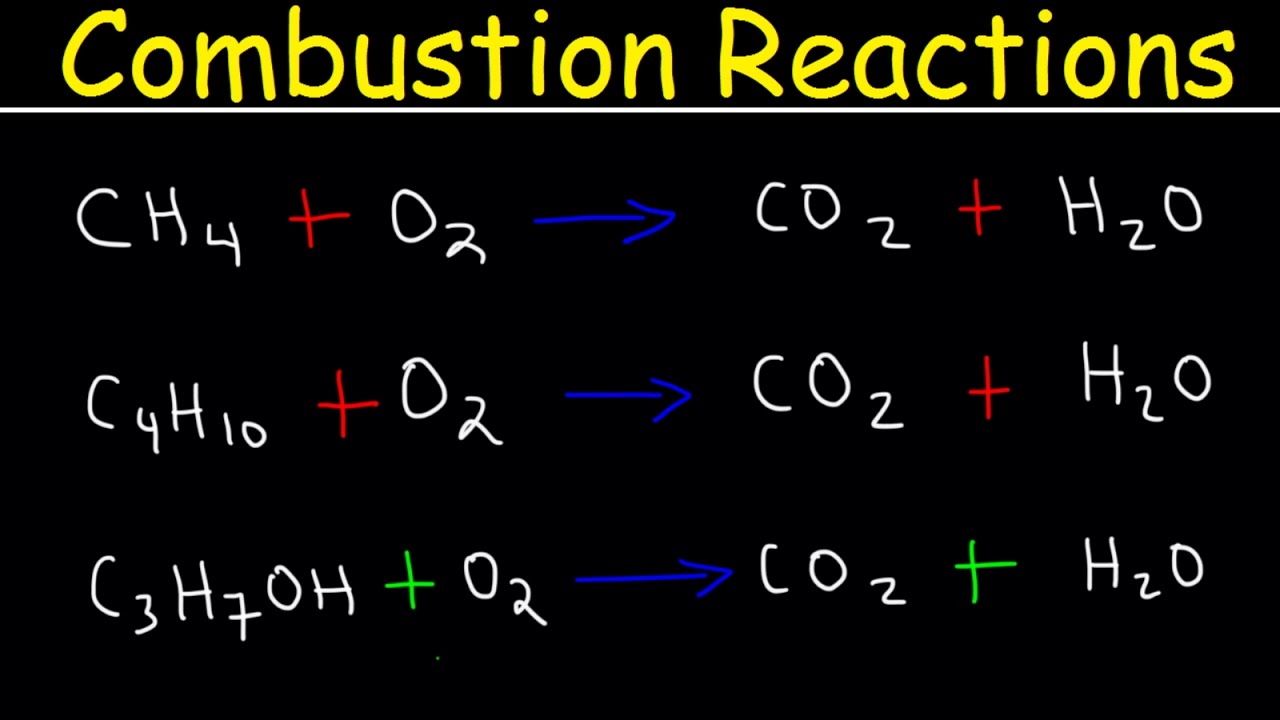
This paragraph introduces the concept of combustion, which is the process of burning a substance with oxygen, typically initiated by a spark. It emphasizes the importance of hydrocarbons, which are molecules composed of hydrogen and carbon, in combustion reactions. The paragraph explains that when hydrocarbons react with oxygen (O2), the result is always the formation of carbon dioxide and water, regardless of the specific hydrocarbon involved. The video aims to teach viewers how to predict these products and distinguish combustion reactions from other types of chemical reactions, such as single replacement reactions.
🧐 Identifying Combustion Reactions and Predicting Products
The second paragraph focuses on how to identify whether a given reaction is a combustion reaction by checking for the presence of a hydrocarbon (a molecule containing only carbon and hydrogen) and its interaction with oxygen gas (O2). It provides examples of different reactions, explaining which are combustion reactions and which are not, based on the reactants involved. The paragraph also clarifies that combustion reactions always result in carbon dioxide and water, and this is not a single replacement reaction despite the presence of an element and a compound. The goal is to practice recognizing combustion reactions and predicting their products, which are consistently carbon dioxide and water, before moving on to balance the chemical equations.
Mindmap
Keywords
💡Combustion
💡Hydrocarbon
💡Oxygen Gas
💡Carbon Dioxide
💡Water
💡Spark
💡Single Replacement
💡Double Replacement
💡Balancing Equations
💡Reactants
💡Products
Highlights
Combustion is defined as starting something on fire by burning it with oxygen and a spark.
Hydrocarbons are compounds consisting of hydrogen and carbon, which are key in combustion reactions.
Combustion involves a hydrocarbon reacting with oxygen gas, often symbolized as O2, and a spark to initiate the fire.
The presence of oxygen is crucial for combustion to occur; without it, the fire cannot sustain.
Every combustion reaction, regardless of the hydrocarbon involved, produces carbon dioxide and water.
Recognizing a hydrocarbon is essential for predicting the products of a combustion reaction.
Combustion is not a single replacement reaction, despite sometimes appearing similar when only considering the reactants.
The products of a combustion reaction are always carbon dioxide and water, regardless of the hydrocarbon's specific composition.
The process of determining if a reaction is a combustion reaction involves checking for a hydrocarbon and its interaction with oxygen.
Examples of hydrocarbons include methane (CH4) and propane (C3H8), which are simple forms consisting of carbon and hydrogen.
The video provides a method to differentiate between combustion and single replacement reactions by analyzing the reactants.
For practice, the video presents several reaction equations to identify which are combustion reactions.
The video emphasizes the importance of recognizing that combustion reactions always result in carbon dioxide and water, regardless of the hydrocarbon.
Balancing the chemical equations for combustion reactions is important but the focus of the video is on predicting the products, not balancing.
The video concludes with practice problems to reinforce the understanding of identifying and predicting products for combustion reactions.
It is clarified that not all reactions involving a hydrocarbon are combustion reactions; the presence of oxygen is a determining factor.
The video instructs viewers to pause and attempt the practice problems independently before revealing the answers.
Transcripts
Browse More Related Video
Balancing Combustion Reactions

Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems

Chemistry Lesson: Types of Chemical Reactions

Chemical Reactions (3 of 11) Combustion Reactions, An Explanation

Classifying Types of Chemical Reactions Practice Problems

5 Types of Chemical Reactions (Chemistry) + Activity Series, Solubility Rules
5.0 / 5 (0 votes)
Thanks for rating: