Quick Revision - Alkanes
TLDRThis educational video delves into the fundamental properties of alkanes, which are saturated hydrocarbons with single carbon-carbon bonds. It explains their tetrahedral geometry, characterized by a 109.5-degree bond angle, and how this structure facilitates free rotation. The video also covers the increasing boiling points of straight-chain alkanes due to stronger intermolecular forces, contrasting with the decrease in boiling points for branched isomers. It touches on alkanes' unreactivity due to their strong carbon-carbon and carbon-hydrogen bonds, low polarity, and similar electronegativities. The script further explores alkanes' reactions with oxygen for combustion and with halogens under UV light, illustrating the radical substitution mechanism involved in these reactions. The summary highlights the challenges in using these reactions for synthesizing single organic compounds due to the potential for multiple substitutions.
Takeaways
- 🌐 Alkanes are hydrocarbons composed solely of carbon and hydrogen atoms.
- 🔗 They are saturated, meaning they contain only single carbon-carbon bonds.
- 🧬 All atoms in alkanes are connected by sigma bonds, allowing free rotation.
- 📐 The shape around each carbon atom in an alkane is tetrahedral, with a bond angle of 109.5 degrees.
- 🔝 The boiling points of straight-chain alkanes increase with the length of the carbon chain due to stronger intermolecular forces.
- 🌿 Branched isomers of alkanes have lower boiling points as increased branching weakens intermolecular forces.
- 🔥 Alkanes are generally unreactive due to their strong carbon-carbon and carbon-hydrogen bonds and low polarity.
- 🚗 They can undergo combustion in the presence of oxygen, producing carbon dioxide and water, making them suitable as fuels.
- ⚠️ Incomplete combustion of alkanes can lead to the production of toxic carbon monoxide and soot.
- 🌞 Alkanes react with halogens under UV light through a radical substitution mechanism.
- 🔬 The radical substitution reaction can lead to multiple products, making it an inefficient method for synthesizing a single organic compound.
Q & A
What are alkanes and what elements do they primarily consist of?
-Alkanes are hydrocarbons that contain only carbon and hydrogen atoms.
What is the nature of the bonds in alkanes?
-Alkanes have single carbon-carbon bonds, which are saturated bonds. All atoms in alkanes are bonded via sigma bonds, allowing free rotation.
What is the shape around each carbon atom in an alkane?
-The shape around each carbon atom in an alkane is tetrahedral with a bond angle of 109.5 degrees.
How do the boiling points of straight chain alkanes relate to the length of their carbon chain?
-The boiling points of straight chain alkanes increase as the carbon chain gets longer due to stronger induced dipole-dipole interactions or London forces.
Why do branched isomers of alkanes have lower boiling points compared to their straight chain counterparts?
-Branched isomers have lower boiling points because the degree of branching reduces the induced dipole-dipole interactions and surface contact, leading to weaker intermolecular forces.
Why are alkanes generally unreactive?
-Alkanes are unreactive due to their high bond enthalpies and low polarity, resulting from the similar electronegativities of carbon and hydrogen.
What happens when alkanes combust in a plentiful supply of oxygen?
-Alkanes combust completely in a plentiful supply of oxygen to produce carbon dioxide and water, which is an exothermic reaction.
What are the products of incomplete combustion of alkanes?
-Incomplete combustion of alkanes can produce carbon monoxide, carbon (soot), and water. Carbon monoxide is toxic and reduces the blood's ability to carry oxygen.
How do alkanes react with halogens, and what is required for this reaction to occur?
-Alkanes react with halogens through a radical substitution mechanism, which requires the presence of UV light due to the strength of their non-polar bonds.
What is the significance of the radical substitution mechanism in the reaction between alkanes and halogens?
-The radical substitution mechanism allows for the substitution of hydrogen atoms with halogen atoms on the alkane. However, it can lead to multiple products and further substitutions if halogens are in excess, making it not ideal for synthesizing a single organic compound.
What are the three stages of the radical substitution mechanism in the reaction between alkanes and halogens?
-The three stages are initiation, where UV light breaks the halogen bond to form radicals; propagation, where radicals react with the alkane and more radicals are formed; and termination, where two radicals combine to form a non-radical substance.
Outlines
🌌 Basic Properties and Reactivity of Alkanes
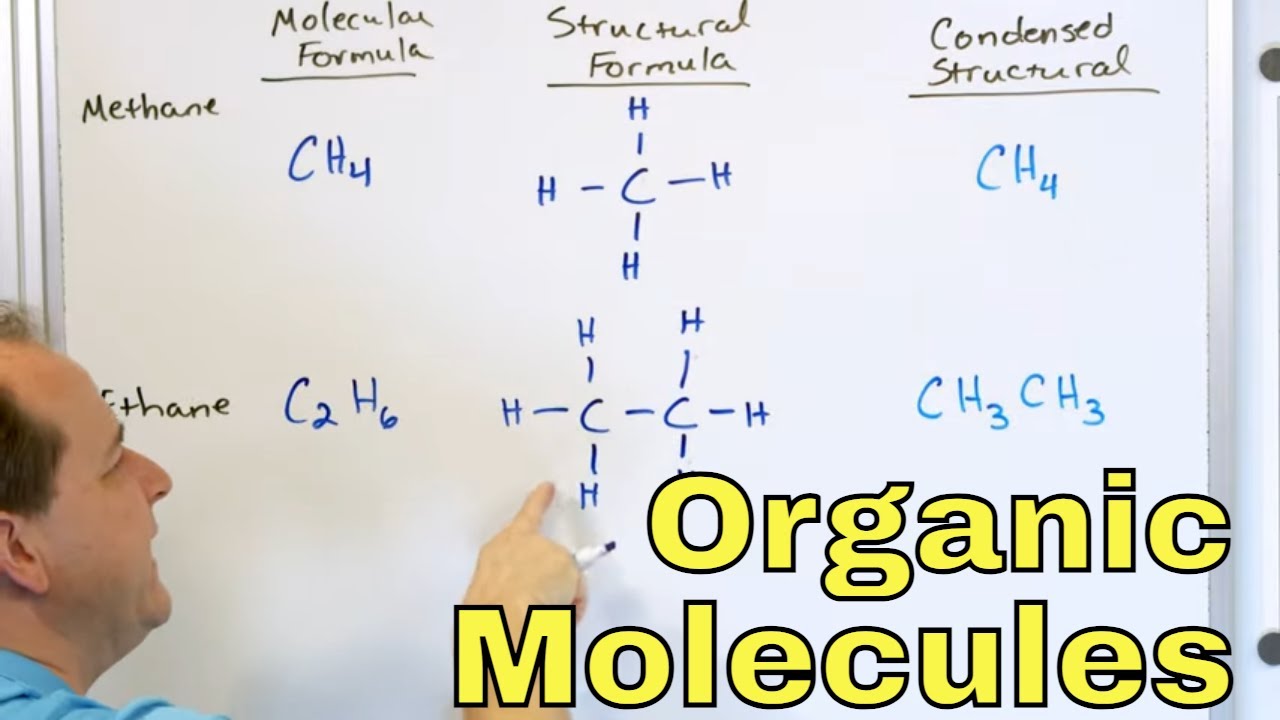
This paragraph introduces alkanes as saturated hydrocarbons containing only carbon and hydrogen atoms, with single carbon-carbon bonds. The electron orbitals overlap directly, forming sigma bonds that allow free rotation. The spatial arrangement around each carbon atom is tetrahedral with a 109.5-degree bond angle, influenced by the four bonding pairs of electrons. The boiling points of straight-chain alkanes increase with chain length due to stronger induced dipole-dipole interactions or London forces. Branched isomers have lower boiling points due to weaker interactions and reduced surface contact. Alkanes are generally unreactive due to their strong carbon-carbon and carbon-hydrogen bonds with low polarity, stemming from the similar electronegativities of carbon and hydrogen.
🔥 Combustion and Halogenation Reactions of Alkanes
The paragraph discusses alkanes' reactivity, highlighting their ability to combust completely in oxygen, producing carbon dioxide and water, making them suitable as fuels. The example of octane, a main hydrocarbon in petrol, is used to illustrate the balanced combustion equation. Incomplete combustion, resulting from insufficient oxygen, leads to the formation of toxic carbon monoxide or soot, which are respiratory irritants. Alkanes also react with halogens like chlorine or bromine under UV light through a radical substitution mechanism. This reaction can lead to multiple substitution products, making it an inefficient method for synthesizing a single organic compound. The mechanism involves three stages: initiation by UV light breaking the halogen bond, propagation involving radical reactions with hydrogen and halogen molecules, and termination where radicals combine to form non-radical substances. Excess halogens can lead to further substitutions, resulting in various chlorinated products.
Mindmap
Keywords
💡Alkanes
💡Saturated Hydrocarbons
💡Sigma Bonds
💡Tetrahedral Geometry
💡Boiling Points
💡Induced Dipole-Dipole Interactions
💡Branched Isomers
💡Reactivity
💡Combustion
💡Halogenation
💡Radical Substitution Mechanism
Highlights
Alkanes are saturated hydrocarbons containing only carbon and hydrogen atoms.
Alkanes have single carbon-carbon bonds and all atoms are bonded via sigma bonds allowing free rotation.
The shape around each carbon atom in an alkane is tetrahedral with a 109.5-degree bond angle.
Methane serves as an example of alkane structure, with a 3D representation illustrating its spatial arrangement.
Boiling points of straight chain alkanes increase with the length of the carbon chain due to stronger intermolecular forces.
Branched isomers of alkanes have lower boiling points due to weaker induced dipole-dipole interactions and reduced surface contact.
Alkanes are typically unreactive due to high bond enthalpies and low polarity of carbon-carbon and carbon-hydrogen bonds.
Alkanes can undergo combustion in the presence of oxygen, producing carbon dioxide and water, making them suitable as fuels.
Incomplete combustion of alkanes can lead to the formation of toxic carbon monoxide and soot particulates.
Alkanes react with halogens like chlorine or bromine under UV light through a radical substitution mechanism.
The reaction between ethane and chlorine under UV light produces chloroethane and hydrogen chloride.
Propane reacts with chlorine to form chloropropane, with the possibility of substitution on different carbon atoms.
Multiple substitutions can occur if halogens are in excess, leading to the formation of dichloropropane and other substituted products.
The radical substitution mechanism involves three stages: initiation, propagation, and termination.
Initiation involves UV light breaking the covalent bond in chlorine to form two chlorine radicals.
Propagation steps involve radicals reacting with alkanes and halogens to form new radicals and non-radical substances.
Termination occurs when two radicals combine to form a non-radical substance, such as chlorine or chloroethane.
Excess halogens can lead to further substitutions, resulting in the formation of dichloro and higher substituted alkanes.
Transcripts
Browse More Related Video

GCSE Chemistry - Alkanes: properties & combustion #52

Hydrocarbons | #aumsum #kids #science #education #children

Physical Properties of Alkanes - Melting Point, Boiling Point, Density, & Water Solubility

12.2 Properties of Alcohols | Organic Chemistry

AP Chemistry multiple choice sample: Boiling points
Visualize & Name Organic Compounds in Organic Chemistry - [1-2-32]
5.0 / 5 (0 votes)
Thanks for rating: