Chapter 6: K and Standard Gibbs Energy | CHM 214 | 051
TLDRThe video script delves into the connection between equilibrium and thermodynamic variables, emphasizing the concept of standard values for enthalpy, entropy, and Gibbs free energy. It explains that these values are determined at a pressure of one bar and at standard states of substances. The script highlights the significance of the standard Gibbs free energy change (ΔG°) in determining the equilibrium constant (K) for a reaction, as expressed by the equation K = e^(-ΔG°/RT). It clarifies that K indicates the spontaneity of a reaction at equilibrium, with K > 1 for spontaneous processes and K < 1 for non-spontaneous ones, and notes that the temperature must be in Kelvin. The discussion sets the stage for exploring reactions not at equilibrium in subsequent content.
Takeaways
- 📈 The concept of standard values is crucial for understanding the relationship between equilibrium and thermodynamic variables.
- 🔍 Standard values for enthalpy, entropy, and Gibbs free energy can be found in tables for various reactions.
- 🌡️ The standard state is defined as the condition where all reactants and products are at a pressure of one bar (approximately one atmosphere).
- 🎓 The standard state also refers to the physical form of substances at room temperature, such as gases for gases, dissolved in solution for aqueous substances, and pure forms for liquids and solids.
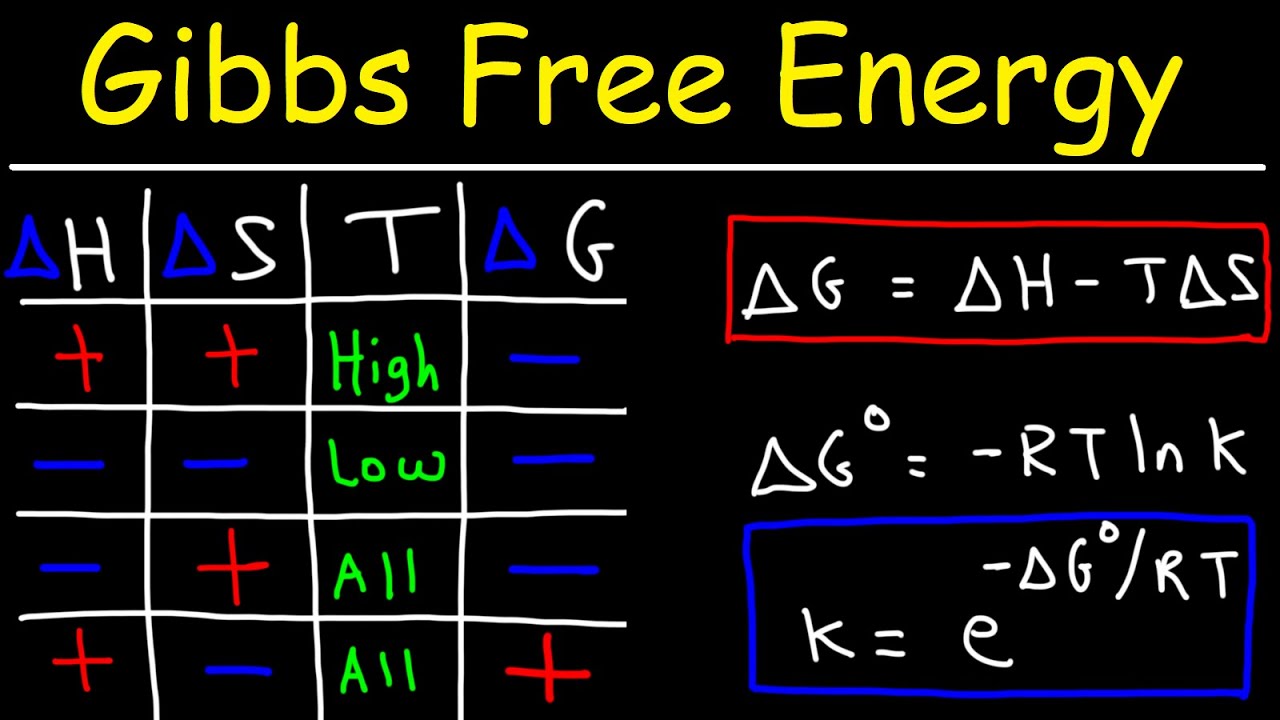
- 📊 The notation for standard enthalpy, entropy, and Gibbs free energy includes a degree symbol next to the delta (Δ) symbol, e.g., ΔH°, ΔS°, ΔG°.
- 🔗 The equilibrium constant (K) for a reaction is related to the standard change in Gibbs free energy (ΔG°) through the equation K = exp(-ΔG° / RT), where R is the ideal gas constant and T is the temperature in Kelvin.
- 🔑 The ideal gas constant (R) is 8.3145 joules per mole times Kelvin, and it can be expressed in various units.
- ⚖️ The value of ΔG° determines the spontaneity of a reaction; if ΔG° is negative, the reaction is spontaneous (K > 1), and if ΔG° is positive, the reaction is non-spontaneous (K < 1).
- 🌟 The equilibrium constant (K) only provides information about systems that are already at equilibrium.
- 🚀 Future discussions will explore how to predict the direction of reactions that are not initially at equilibrium, such as when substances are simply mixed together.
Q & A
What are standard values in the context of thermodynamics and chemical reactions?
-Standard values refer to the specific enthalpy, entropy, and Gibbs free energy values for a chemical reaction under standard conditions, which include all reactants and products being in their standard states at a pressure of one bar, typically near one atmosphere.
How is the standard state defined for a substance?
-The standard state for a substance is defined based on its physical and chemical state under standard conditions. For example, hydrogen is in a gaseous form, while substances that are aqueous are dissolved in a solution at a concentration of one molar.
What is the notation used to denote standard enthalpy, entropy, and Gibbs free energy?
-The notation for standard enthalpy, entropy, and Gibbs free energy includes a degree symbol next to the respective variable, such as ΔH° for standard enthalpy, ΔS° for standard entropy, and ΔG° for standard Gibbs free energy.
What is the significance of standard conditions in determining the equilibrium constant for a reaction?
-Standard conditions are crucial in determining the equilibrium constant (K) for a reaction because they provide a consistent basis for comparison. The equilibrium constant is related to the standard change in Gibbs free energy (ΔG°), which is used to predict the spontaneity of a reaction under standard conditions.
How is the equilibrium constant (K) calculated from the standard Gibbs free energy change (ΔG°)?
-The equilibrium constant (K) is calculated using the equation K = exp(-ΔG° / (R * T)), where R is the ideal gas constant (8.3145 J/mol·K), T is the temperature in Kelvin, and exp denotes the exponential function.
What does the value of K indicate about a reaction's spontaneity?
-If ΔG° is negative, indicating an exothermic reaction, K will be greater than one, signifying that the reaction is spontaneous under standard conditions. Conversely, if ΔG° is positive, K will be less than one, indicating a non-spontaneous reaction under standard conditions.
Why is it important to use Kelvin when calculating the equilibrium constant (K)?
-Temperature must be in Kelvin when calculating K because the ideal gas constant (R) and the exponential function in the equation for K are based on the Kelvin scale. Using other temperature scales would lead to incorrect calculations.
What happens when a reaction is not at equilibrium?
-When a reaction is not at equilibrium, the system will continue to shift towards the equilibrium position, either towards the products or reactants, depending on the initial conditions and the direction of the reaction.
How can we predict the direction a reaction will go if it's not initially at equilibrium?
-We can predict the direction a reaction will go by considering the initial concentrations or pressures of reactants and products, along with their respective standard Gibbs free energy changes (ΔG°), and using additional thermodynamic data such as enthalpy and entropy changes.
What is the role of the ideal gas constant (R) in the calculation of the equilibrium constant (K)?
-The ideal gas constant (R) is a fundamental constant used in the calculation of K, as it relates the Gibbs free energy change (ΔG°) to the equilibrium constant through the equation K = exp(-ΔG° / (R * T)). It ensures the correct scaling of the energy units and temperature in the calculation.
How does the standard enthalpy change of formation (ΔH°f) relate to the energy content of a compound?
-The standard enthalpy change of formation (ΔH°f) represents the enthalpy change when one mole of a compound is formed from its elements in their standard states. It provides a measure of the relative energy content of the compound compared to its constituent elements.
Why is the standard enthalpy change of formation for an element in its standard state considered to be zero?
-The standard enthalpy change of formation for an element in its standard state is zero because there is no change in the energy content when an element is formed from itself. The definition of enthalpy change of formation inherently assumes no energy change when a substance is transformed from its elements to itself.
Outlines
📚 Introduction to Standard Values in Thermodynamics
This paragraph introduces the concept of standard values in the context of thermodynamics, emphasizing their importance in understanding and calculating enthalpy, entropy, and Gibbs free energy for chemical reactions. It explains that standard values are referenced from tables and are defined under specific conditions where all reactants and products are in their standard state at a pressure of one bar. The standard state varies depending on the substance, with gases like hydrogen being in their gaseous form, aqueous substances dissolved in solution at a standard molar concentration, and pure liquids and solids being in their pure states.
Mindmap
Keywords
💡Equilibrium
💡Thermodynamic Variables
💡Standard Values
💡Enthalpy
💡Entropy
💡Gibbs Free Energy
💡Standard State
💡Equilibrium Constant
💡Ideal Gas Constant
💡Temperature in Kelvin
💡Spontaneous Reaction
Highlights
Link between equilibrium and thermodynamic variables discussed.
Standard values for enthalpy, entropy, and Gibbs free energy are essential for reactions.
Standard values can be found in tables for various reactions.
The standard state is defined for reactants and products at a pressure of one bar.
Standard state means substances are in their typical form at room temperature.
Hydrogen in gaseous form is the standard state for hydrogen.
Aqueous substances are in solution and at a standard state of one molar.
Pure liquids and solids are also in their standard states.
Equilibrium is related to delta G (Gibbs free energy).
The relationship between equilibrium constant (K) and standard Gibbs free energy change (Delta G naught) is given by the equation K = e^(-Delta G naught / R * T).
R is the ideal gas constant (8.3145 J/(mol*K)).
Temperature must be in Kelvin, not Celsius or Fahrenheit.
If Delta G naught is negative, the reaction is spontaneous and K > 1.
If Delta G naught is positive, the reaction is non-spontaneous and K < 1.
The equilibrium constant K only provides information about systems at equilibrium.
Future discussions will explore determining reaction direction for non-equilibrium systems.
Transcripts
Browse More Related Video

6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant | Organic Chemistry
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry

Gibbs free energy and spontaneous reactions | Biology | Khan Academy

Chapter 6: Gibbs Free Energy | CHM 214 | 050
[H2 Chemistry] 2021 Topic 5 Energetics 3
5.0 / 5 (0 votes)
Thanks for rating: