Trick to Calculate Molarity | Molarity practice problems
TLDRThis educational video script outlines a simple method for calculating molarity, a measure of the number of moles of solute per liter of solution. It explains the basic formula (m = n/V) and provides step-by-step examples using different scenarios, including converting mass to moles and volume from milliliters to liters. The script also emphasizes the importance of understanding volume conversions and offers a trick to easily convert milliliter volumes to liters by moving the decimal point three places to the left. The examples cover various substances like HCL, sodium chloride, and sodium hydroxide, illustrating how to apply the formula to find molarity in different situations.
Takeaways
- 🧪 Molarity is defined as the number of moles of solute dissolved in 1 liter of solution.
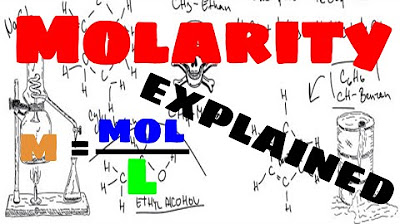
- 📏 The formula for molarity is M = n/V, where M is molarity, n is moles of solute, and V is volume in liters.
- 🌡️ An example given is dissolving 6 moles of HCL in 1 liter of water, resulting in a 6 M molarity.
- 📐 Conversion from milliliters or cubic centimeters to liters is necessary, by moving the decimal point three places to the left.
- 🔢 For a 0.5 mole of sodium chloride in 400 ml of water, the molarity is calculated to be 1.25 M.
- 🧪 For 0.9 mole of HCL in 500 cm³ of water, the molarity is 1.8 M after converting volume to liters.
- 📉 For 0.2 mole of HNO3 in 200 ml of water, the molarity is found to be 1 M after conversion.
- 🔍 When the mass of a solute is given, it must be converted to moles using the molar mass of the solute.
- ⚗️ An example with 2 grams of sodium hydroxide is used, where the molar mass calculation leads to a 0.05 mole determination.
- 📐 The volume conversion for the sodium hydroxide solution from 600 cm³ to 0.6 liters is essential for molarity calculation.
- 📊 The molarity of the sodium hydroxide solution is calculated to be 0.083 M using the formula M = n/V.
- 🧪 For more complex problems, the molar mass of the solute must be calculated and used to find the number of moles.
Q & A
What is molarity and how is it defined?
-Molarity is defined as the number of moles of solute dissolved in 1 liter of solution. It is a measure of the concentration of a solute in a solution.
What is the formula used to calculate molarity?
-The formula to calculate molarity is m = n/V, where 'm' is the molarity of the solution, 'n' is the number of moles of solute, and 'V' is the volume of the solution in liters.
How many moles of HCL are needed to make a 6 M molarity solution in a 1-liter solution?
-To make a 6 M molarity solution, you would need 6 moles of HCL dissolved in 1 liter of solution.
What is the relationship between liters, decimeters cubed (dm³), centimeters cubed (cm³), and milliliters?
-One liter is equal to 1 decimeter cubed (dm³), which is also equal to 1000 centimeters cubed (cm³) or 1000 milliliters.
How do you convert the volume of a solution from milliliters to liters?
-To convert the volume from milliliters to liters, you move the decimal point three places to the left or divide the volume in milliliters by 1000.
What is the molarity of a solution if 0.5 moles of sodium chloride are dissolved in 400 ml of water?
-The molarity of the solution would be 1.25 M, calculated by converting 400 ml to 0.4 liters and using the formula m = n/V, where n = 0.5 moles and V = 0.4 liters.
How do you calculate the molarity of a solution when the mass of the solute is given instead of the number of moles?
-First, you need to find the molar mass of the solute and then convert the given mass to the number of moles by dividing the mass by the molar mass. After that, use the molarity formula m = n/V with the calculated number of moles and the volume in liters.
What is the molar mass of sodium hydroxide (NaOH) and how do you find the number of moles from a given mass?
-The molar mass of sodium hydroxide (NaOH) is 40 grams (23 for sodium, 16 for oxygen, and 1 for hydrogen). To find the number of moles from a given mass, divide the mass of NaOH by its molar mass.
How would you calculate the molarity of a solution with 2 grams of sodium hydroxide dissolved in 600 cm³ of water?
-First, convert the 600 cm³ to liters by moving the decimal point three places to the left, which gives 0.6 liters. Then, calculate the number of moles of NaOH (0.05 moles from 2 grams) and use the formula m = n/V to find the molarity, which would be 0.083 M.
What is the molar mass of HNO₃ and how do you calculate the molarity of a solution with 4 grams of HNO₃ in 80 ml of water?
-The molar mass of HNO₃ is 63 grams (1 for hydrogen, 14 for nitrogen, and 16 * 3 for oxygen). To calculate the molarity, convert 80 ml to 0.08 liters, find the number of moles (0.06 moles from 4 grams), and use the formula m = n/V to get a molarity of 0.75 M.
How do you find the molarity of a solution with a given mass of HCl and a specific volume?
-Find the molar mass of HCl (36.5 grams), calculate the number of moles from the given mass (0.55 moles from 20 grams), and use the volume in liters (0.5 liters from 0.5 dm³) with the formula m = n/V to find a molarity of 1.1 M.
Outlines
🧪 Understanding and Calculating Molarity
This paragraph introduces the concept of molarity, which is the measure of the number of moles of solute dissolved in one liter of solution. The formula for molarity (m = n/V) is explained, where 'm' is molarity, 'n' is the number of moles, and 'V' is the volume in liters. An example is given where 6 moles of HCL are dissolved in 1 liter of water, resulting in a 6 M solution. The paragraph also explains the importance of converting volumes from milliliters or cubic centimeters to liters by moving the decimal point three places to the left. Several numerical problems are solved to demonstrate the calculation of molarity, including scenarios where the volume of the solution is given in different units and where the mass of the solute is provided instead of the number of moles.
📚 Advanced Molarity Calculations with Mass and Volume Conversions
The second paragraph delves into more complex molarity problems where the mass of the solute is given, requiring the calculation of the number of moles before determining molarity. Examples include converting 2 grams of sodium hydroxide (with a molar mass of 40 grams/mole) into 0.05 moles and then calculating the molarity in a 600 cm³ solution, which is 0.083 M. Similarly, the molar mass of HNO₃ (63 grams/mole) is used to convert 4 grams of HNO₃ into 0.06 moles, and its molarity in 80 ml of solution is found to be 0.75 M. The paragraph concludes with a challenge problem involving HCl, where the molar mass (36.5 grams/mole) is used to convert 20 grams into 0.55 moles, and the molarity of the solution in 0.5 DM³ (equivalent to 0.5 liters) is calculated to be 1.1 M. The paragraph emphasizes the importance of unit conversions and the use of the molarity formula in various scenarios.
Mindmap
Keywords
💡Molarity
💡Moles
💡Solute
💡Solution
💡Volume
💡Milliliter
💡Cubic Centimeter
💡Molar Mass
💡Concentration
💡Numerical Problems
Highlights
Molarity is defined as the number of moles of solute dissolved in 1 liter of solution.
The formula for molarity is m = n/V, where m is molarity, n is moles of solute, and V is volume of solution.
Example given: 6 moles of HCL dissolved in 1 liter of solution results in a 6 M molarity.
1 liter is equivalent to 1 cubic decimeter (DM cube), which is equal to 1000 cubic centimeters (CM cube) or 1000 milliliters.
To calculate molarity, convert the volume of solution from milliliters to liters by moving the decimal point three places to the left.
Example: 0.5 moles of sodium chloride in 400 ml (0.4 liters) results in a 1.25 M molarity.
Example: 0.9 moles of HCL in 500 cm cube (0.5 liters) results in a 1.8 M molarity.
Example: 0.2 moles of HNO3 in 200 ml (0.2 liters) results in a 1 M molarity.
When given mass of solute, convert it to moles using the molar mass before calculating molarity.
Example: 2 grams of sodium hydroxide (molar mass 40 grams) results in 0.05 moles.
Example: 0.05 moles of sodium hydroxide in 600 cm cube (0.6 liters) results in a 0.083 M molarity.
Molar mass of HNO3 is calculated as 1 (H) + 14 (N) + 3*16 (O) = 63 grams.
Example: 4 grams of HNO3 (molar mass 63 grams) results in 0.06 moles.
Example: 0.06 moles of HNO3 in 80 ml (0.08 liters) results in a 0.75 M molarity.
Molar mass of HCL is calculated as 1 (H) + 35.5 (Cl) = 36.5 grams.
Example: 20 grams of HCL (molar mass 36.5 grams) results in 0.55 moles.
Example: 0.55 moles of HCL in 0.5 DM cube (0.5 liters) results in a 1.1 M molarity.
Transcripts
Browse More Related Video

Molarity Practice Problems

Molarity Made Easy: How to Calculate Molarity and Make Solutions

Molarity Practice Problems
Molarity Explained

Concentration and Molarity explained: what is it, how is it used + practice problems

How to Do Solution Stoichiometry Using Molarity as a Conversion Factor | How to Pass Chemistry
5.0 / 5 (0 votes)
Thanks for rating: