Molarity Explained
TLDRIn this educational video, Mr. Millings introduces molarity, a fundamental concept in chemistry for calculating the concentration of a solution. He explains that molarity is the number of moles of solute per liter of solution, simplifying the process compared to mass percent. Through step-by-step examples, he demonstrates how to calculate molarity, convert between moles, liters, and molarity, and apply these concepts to various problems. The video covers practical problems involving different substances, emphasizing the importance of molarity in scientific and chemical contexts.
Takeaways
- 🧪 Molarity is a method used to calculate the concentration of a solution, which is preferred by chemists and scientists over mass percent.
- 📐 The formula for molarity (M) is the moles of solute (n) divided by the liters of solution (V): M = n/V.
- 📦 To find molarity, you divide the total moles of the solute by the total liters of the solution.
- 🔄 From the molarity formula, three different formulas can be derived to solve for moles, liters, or molarity itself.
- 📘 To calculate molarity, you might need to convert grams of a substance to moles using the molar mass.
- 💧 When dealing with volumes, it's often necessary to convert milliliters to liters by moving the decimal three places to the left.
- 📊 Molarity provides a measure of how many moles of solute are present per liter of solution, indicating the solution's concentration.
- 📚 The video provides step-by-step examples to demonstrate how to calculate molarity and related quantities.
- 📉 Molarity can be used to determine how concentrated a solution is, with higher molarity indicating a more concentrated solution.
- 🔢 The script includes several practice problems to illustrate the application of molarity in calculating solution concentrations.
- 🧐 Understanding molarity is crucial for solving chemistry problems that involve the concentration of solutions.
Q & A
What is molarity and how is it used to measure the concentration of a solution?
-Molarity is a measure of the concentration of a solution, expressed as the number of moles of solute per liter of solution. It is used to calculate the concentration by dividing the moles of dissolved solute by the total liters of the solution.
How is molarity different from mass percent?
-Molarity focuses on the amount of solute in terms of moles per liter of solution, whereas mass percent calculates the concentration as the mass of the solute divided by the total mass of the solution, multiplied by 100.
What is the formula for calculating molarity?
-The formula for calculating molarity (M) is M = moles of solute / liters of solution.
If you have 2.5 moles of salt dissolved in 0.5 liters of water, what is the molarity of the saltwater solution?
-The molarity of the saltwater solution would be 5 moles per liter (M = 2.5 moles / 0.5 liters).
What does a molarity of 5 moles per liter mean?
-A molarity of 5 moles per liter means that for every liter of the solution, there are 5 moles of the solute (in this case, sodium chloride) dissolved in it.
How can you find the moles of solute in a solution if you know the molarity and the volume of the solution?
-You can find the moles of solute by multiplying the volume of the solution (in liters) by the molarity (in moles per liter).
How can you determine the volume of a solution if you know the moles of solute and the molarity?
-To determine the volume of the solution, divide the moles of solute by the molarity (M = moles of solute / liters of solution).
In the example problem with 255 moles of salt dissolved in 3.75 liters of solution, what is the molarity of the solution?
-The molarity of the solution is 0.680 moles per liter (M = 255 moles / 3.75 liters).
If a solution has a molarity of 1.35 x 10^-4 M and you have 25.5 liters of it, how many moles of solute are dissolved in it?
-The number of moles of solute dissolved in the solution is 3.44 x 10^-3 moles (moles = 25.5 liters * 1.35 x 10^-4 M).
How do you calculate the molarity of a solution containing 705 grams of Al2(SO4)3 in 345 milliliters of water?
-First, convert the grams of Al2(SO4)3 to moles using its molar mass, and convert the milliliters of water to liters. Then, divide the moles of solute by the liters of solution to find the molarity.
What is the molar mass of Al2(SO4)3 and how do you calculate it?
-The molar mass of Al2(SO4)3 is 342 grams per mole, calculated by adding the molar mass of aluminum (2 * 27 g/mol), sulfur (3 * 32 g/mol), and oxygen (12 * 16 g/mol).
If you have 705 grams of Al2(SO4)3 and 345 milliliters of water, what is the molarity of the resulting solution?
-The molarity of the solution is 0.597 M (moles of Al2(SO4)3 / liters of solution = 0.206 moles / 0.345 liters).
Outlines
🧪 Introduction to Molarity
In this introductory segment, Mr. Millings explains the concept of molarity, a method used by chemists to calculate the concentration of a solution. He contrasts it with mass percent, which was covered in a previous video, and introduces the formula for molarity: moles of solute divided by liters of solution. An example is given where 2.5 moles of salt are dissolved in 0.5 liters of water to create a solution with a molarity of 5 moles per liter. This indicates that there are five moles of solute for every liter of solution. Mr. Millings also derives three formulas from the molarity equation that can be used to solve for moles, liters, or molarity itself.
📚 Calculating Molarity with Given Moles and Liters
This paragraph demonstrates how to calculate the molarity of a solution when the moles of solute and the volume of the solution in liters are provided. An example problem is solved where a saltwater solution contains 2.55 moles of salt in 3.75 liters, resulting in a molarity of 0.680 M. Another example involves a 25.5-liter solution with a molarity of 1.35 x 10^-4 M, and the calculation reveals that 3.44 x 10^-3 moles of solute are dissolved. A third example shows how to find the volume of a solution given its molarity and the moles of solute, resulting in a 4.33-liter solution.
🔍 Determining Solution Volume and Molarity from Solute Mass
The third paragraph involves a more complex problem where the molarity of a solution containing 705 grams of Al2(SO4)3 and 345 milliliters of water needs to be calculated. The solution requires two conversions: grams to moles using the molar mass of aluminum sulfate, and milliliters to liters. After calculating the moles of solute (0.206 moles) and converting the volume of water to liters (0.345 liters), the molarity is found by dividing the moles of solute by the volume of the solution, yielding a molarity of 0.597 M.
📘 Conclusion on Understanding Molarity
In the concluding paragraph, Mr. Millings summarizes the concept of molarity and its importance in chemistry. He emphasizes that molarity provides a measure of how concentrated a solution is, with the example given resulting in a 0.597 M solution, meaning there are 0.597 moles of aluminum sulfate per liter of solution. The paragraph wraps up with a hopeful note that the explanation was helpful for understanding molarity.
Mindmap
Keywords
💡Molarity
💡Concentration
💡Mass Percent
💡Solute
💡Solution
💡Moles
💡Liters
💡Aluminum Sulfate
💡Molar Mass
💡Dilute Solution
Highlights
Introduction to molarity as an easier way to calculate solution concentration compared to mass percent.
Molarity defined as moles of dissolved solute divided by total liters of solution.
Explanation of how to calculate molarity with the formula M = moles of solute / liters of solution.
Example problem: Making a 0.5-liter saltwater solution with 2.5 moles of salt.
Calculation of a five molar solution from the saltwater example.
Derivation of formulas to solve for moles, liters, and molarity from the basic molarity formula.
Practice problem: Calculating molarity of a solution with 2.55 moles of salt in 3.75 liters.
Result of the practice problem showing a 0.680 molar solution.
Second example problem involving a 25.5-liter solution with a molarity of 1.35 x 10^-4 M.
Calculation of moles of solute dissolved in the second example problem.
Third example problem: Determining the volume of a solution with a given molarity and moles of solute.
Solution to the third problem, finding 4.33 liters for a 1.5 M solution with 6.5 moles of solute.
Final example problem: Calculating molarity with 70.5 grams of Al2(SO4)3 and 345 mL of water.
Conversion of grams to moles for Al2(SO4)3 and milliliters to liters for the solution volume.
Final calculation resulting in a 0.597 M solution of aluminum sulfate.
Summary of molarity as a measure of solution concentration with practical application examples.
Transcripts
Browse More Related Video

Molarity Made Easy: How to Calculate Molarity and Make Solutions

How to Calculate Molarity for a Solution

Concentration and Molarity: The Key to Chemical Solutions

How to Do Solution Stoichiometry Using Molarity as a Conversion Factor | How to Pass Chemistry

Trick to Calculate Molarity | Molarity practice problems
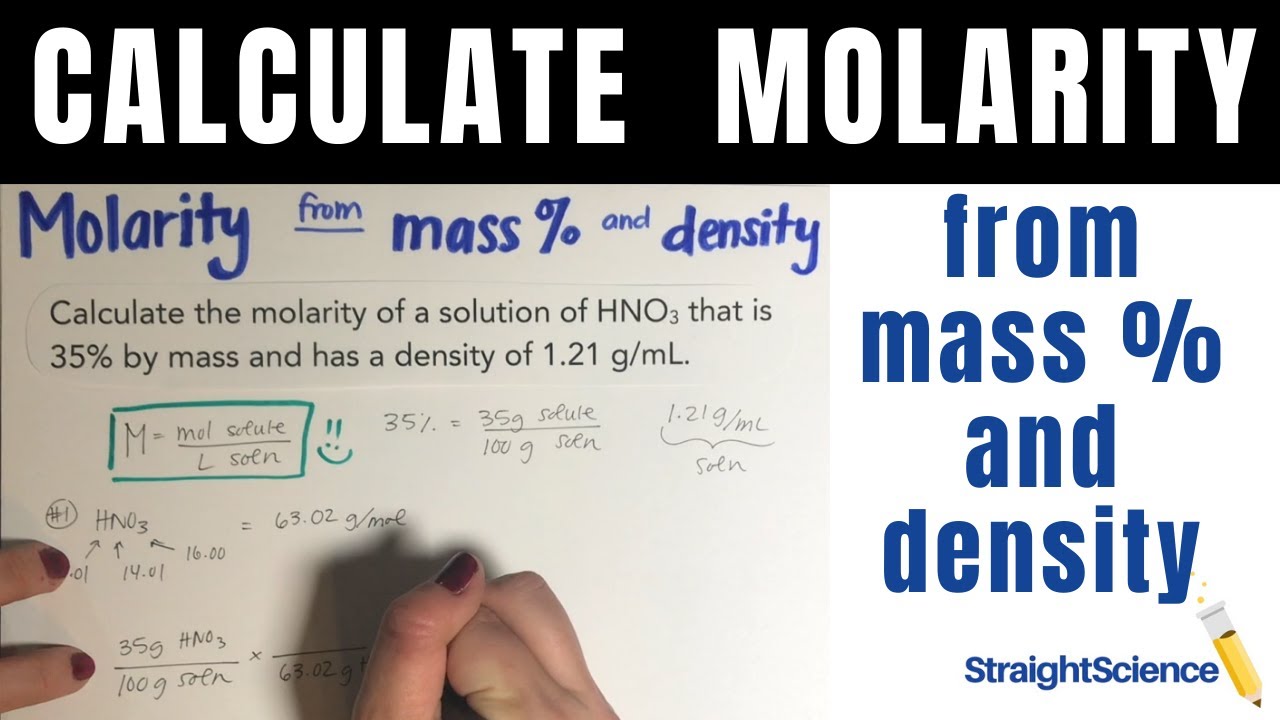
Molarity from Mass % and Density - Calculate Molarity from Mass Percent and Density
5.0 / 5 (0 votes)
Thanks for rating: